Copyright Notice
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

References Used in Algorithms for the Treatment of Persons with Crohn’S Disease
REFERENCES USED IN ALGORITHMS FOR THE TREATMENT OF PERSONS WITH CROHN’S DISEASE 1. AA Pharma Inc: Winpred (prednisone). In: CA Product Monograph. Vaughan, ON; 2018. 2. AbbVie Corporation: Humira (adalimumab). In: CA Product Monograph. St Laurent, QC; 2019. 3. AbbVie Inc: Humira (adalimumab). In: US Product Monograph. North Chicago, IL; 2020. 4. Amgen Canada Inc: Avsola (infliximab). In: CA Product Monograph. Mississauga, ON; 2020. 5. Amgen Inc: Amjevita (adalimumab-atto). In: US Product Monograph. Thousand Oaks, CA; 2019. 6. Amgen Inc: Avsola (infliximab-axxq). In: US Product Monograph. Thousand Oaks, CA; 2019. 7. Antares Pharma Inc: Methotrexate. In: FDA Product Monograph. Ewing, NJ; 2019. 8. Apotex Inc: Methotrexate. In: CA Product Monograph. Toronto, ON; 2019. 9. Asada A, Nishida A, Shioya M, Imaeda H, Inatomi O, Bamba S, Kito K, Sugimoto M, Andoh A: NUDT15 R139C-related thiopurine leukocytopenia is mediated by 6-thioguanine nucleotide-independent mechanism in Japanese patients with inflammatory bowel disease. Journal of gastroenterology 2016, 51(1):22-29. 10. Aspen Pharmacare Canada Inc: Imuran (azathioprine). In: CA Product Monograph. Oakville, ON; 2019. 11. Biogen Canada Inc: Tysabri (natalizumab). In: CA Product Monograph. Mississauga, ON; 2017. 12. Biogen Idec Inc: Tysabri (natalizumab). In: US Product Monograph. Cambridge, MA; 2019. 13. Birdwell KA, Decker B, Barbarino JM, Peterson JF, Stein CM, Sadee W, Wang D, Vinks AA, He Y, Swen JJ et al: Clinical Pharmacogenetics Implementation Consortium (CPIC) Guidelines for CYP3A5 Genotype and Tacrolimus Dosing. Clinical pharmacology and therapeutics 2015, 98(1):19-24. 14. Boehringer Ingelheim Pharmaceuticals Inc: Cyltezo (adalimumab-adbm). In: US Product Monograph. Ridgefield, CT; 2019. -

Long Term Care Pharmacy
2201812 Long Term Care Pharmacy Long-Term Care Pharmacy of charge as value-added services. They include: Our mission is to provide our long-term care pharmacy › Access to our staff of eight in-house clinical experts. members with the tools they need to maximize savings › CE credits through a monthly teleconference program, while enhancing the quality of care they provide. In 1993, in-person programming, and written education Innovatix began as a pharmacy-focused GPO, and over programs. the years has become the industry leader with over 15,000 › Care Solutions manuals that serve as management unique NDCs and a number of pharmacy support products guides for specific diseases and conditions. and services. As a wholly-owned subsidiary of Premier, › Contract Advantage tools that help members save by Innovatix also offers members access to one of the most providing contracted alternatives to higher-priced non- robust, competitive equipment, supply, and service contracted drugs. portfolios available. › Updated clinical news and resources. GPO Support Services Multi-Tiered Customer Support At Innovatix, we realize that securing best-in-class pricing Innovatix members receive ongoing support from a is only half of the equation. That’s why we’ve developed team of experienced professionals who are committed a suite of tools and services designed to ensure our to delivering exceptional value and complete member members receive discounted pricing and have sufficient satisfaction. Our customer care teams work with each data to make informed purchasing decisions. Our tools member individually, analyzing data to identify purchasing and services include: needs and goals. Our objective is to secure the greatest › Electronic contract attachment technology designed to value for each of our members. -

Pharmaceutical Company Contact Information (PDF)
Pharmaceutical Company Contact Information - Rebate Filing - as of June 2018 Labeler Name Invoice Contact Phone Extension 00002 LILLY USA, LLC LISA NORTON (317) 276-2000 00003 ER SQUIBB AND SONS INC. LYNN LEWIS (609) 897-4731 00004 GENENTECH CONTRACT ADMINISTRATION (650) 866-2666 00005 LEDERLE LABORATORIES DAN MAGUIRE (484) 563-5097 00006 MERCK & CO., INC. DOUG BICKFORD (215) 652-0671 00007 SMITHKLINE BEECHAM DAVID BUCKLEY (215) 751-5690 00008 WYETH LABORATORIES JENNIFER WOOTEN (901) 215-1883 00009 PHARMACIA AND UPJOHN COMPANY/PFIZER JENNIFER WOOTEN (901) 215-1883 00013 PHARMACIA AND UPJOHN COMPANY NICHOLAS CHRISTODOULOU (336) 291-1053 00014 G. D. SEARLE & CO. CINDY MCDONALD (847) 581-5726 00015 MEAD JOHNSON AND COMPANY LYNN LEWIS (609) 897-4731 00016 PHARMACIA INC. BARBARA WINGET (908) 901-7254 00023 ALLERGAN INC SHOBHANA MINAWALA (714) 246-6205 00024 SANOFI WINTHROP PHARMACEUTICALS LAURIE DUNLAP, ADMIN., GOVT. OPERATIONS (212) 551-4198 00025 PHARMACIA CORPORATION NICHOLAS CHRISTODOULOU (336) 291-1053 00026 BAYER CORPORATION PHARMACEUTICAL DIV. LINDA WOLCHESKI (203) 812-6372 00028 NOVARTIS PHARMACEUTICALS (862) 778-8094 00029 SMITHKLINE BEECHAM DAVID BUCKLEY (215) 751-5690 00031 A. H. ROBINS COMPANY DAN MAGUIRE (610) 902-3222 00032 SOLVAY PHARMACEUTICALS STACEY LENOX (847) 937-3979 00033 SYNTEX LABORATORIES, INC. JANICE BRENNAN (973) 562-3494 00034 THE PURDUE FREDERICK COMPANY JUNE STOWE (203) 899-8035 00037 CARTER-WALLACE, INC. JAY R BRENNAN (609) 655-6163 00038 ASTRAZENECA LP DAVID WRIGHT (302) 886-2268 7820 00039 AVENTIS PHARMACEUTICALS (908) 981-7461 00043 NOVARTIS CONSUMER HEALTH, INC. EDWARD D. COLLINS (973) 781-6191 00044 KNOLL LABORATORIES DEBRA DEYOUNG (847) 937-4372 00045 MCNEIL PHARMACEUTICAL (908) 218-6777 00046 AYERST LABORATORIES (901) 215-1473 00047 WARNER CHILCOTT LABORATORIES LISA KAROLCHYK (973) 442-3262 00048 KNOLL PHARMACEUTICAL COMPANY DEBRA DEYOUNG (847) 937-4372 00049 ROERIG NICHOLAS CHRISTODOULOU (336) 291-1053 00051 UNIMED PHARMACEUTICALS, INC STACY LENOX (847) 937-3979 00052 ORGANON, USA, INC. -

Rebateable Manufacturers
Rebateable Labelers – July 2021 Manufacturers are responsible for updating their eligible drugs and pricing with CMS. Montana Healthcare Programs will not pay for an NDC not updated with CMS. Note: Some manufacturers on this list may have some NDCs that are covered and others that are not. Manufacturer ID Manufacturer Name 00002 ELI LILLY AND COMPANY 00003 E.R. SQUIBB & SONS, LLC. 00004 HOFFMANN-LA ROCHE 00006 MERCK & CO., INC. 00007 GLAXOSMITHKLINE 00008 WYETH PHARMACEUTICALS LLC, 00009 PHARMACIA AND UPJOHN COMPANY LLC 00013 PFIZER LABORATORIES DIV PFIZER INC 00015 MEAD JOHNSON AND COMPANY 00023 ALLERGAN INC 00024 SANOFI-AVENTIS, US LLC 00025 PFIZER LABORATORIES DIV PFIZER INC 00026 BAYER HEALTHCARE LLC 00032 ABBVIE INC. 00037 MEDA PHARMACEUTICALS, INC. 00039 SANOFI-AVENTIS, US LLC 00046 WYETH PHARMACEUTICALS INC. 00049 ROERIG 00051 ABBVIE INC 00052 ORGANON USA INC. 00053 CSL BEHRING L.L.C. 00054 HIKMA PHARMACEUTICAL USA, INC. 00056 BRISTOL-MYERS SQUIBB PHARMA CO. 00065 ALCON LABORATORIES, INC. 00068 AVENTIS PHARMACEUTICALS 00069 PFIZER LABORATORIES DIV PFIZER INC 00071 PARKE-DAVIS DIV OF PFIZER 00074 ABBVIE INC 00075 AVENTIS PHARMACEUTICALS, INC. 00078 NOVARTIS 00085 SCHERING CORPORATION 00087 BRISTOL-MYERS SQUIBB COMPANY 00088 AVENTIS PHARMACEUTICALS 00093 TEVA PHARMACEUTICALS USA, INC. 00095 BAUSCH HEALTH US, LLC Page 1 of 19 Manufacturer ID Manufacturer Name 00096 PERSON & COVEY, INC. 00113 L. PERRIGO COMPANY 00115 IMPAX GENERICS 00116 XTTRIUM LABORATORIES, INC. 00121 PHARMACEUTICAL ASSOCIATES, INC. 00131 UCB, INC. 00132 C B FLEET COMPANY INC 00143 HIKMA PHARMACEUTICAL USA, INC. 00145 STIEFEL LABORATORIES, INC, 00168 E FOUGERA AND CO. 00169 NOVO NORDISK, INC. 00172 TEVA PHARMACEUTICALS USA, INC 00173 GLAXOSMITHKLINE 00178 MISSION PHARMACAL COMPANY 00185 EON LABS, INC. -

Convention Exhibitors and Sponsors—October 2007
CONVENTION EXHIBITORS /alert Marketing Drug Enforcement Innovation Outcomes Pharmaceutical Abbott Administration Innovatix HealthCare Abbott Diabetes Care Drug Topics InterCure Owen Mumford Acorda Therapeutics Duramed Pharmaceuticals, Inc. Janssen L.P. PAAS National Adams Respiratory Therapeutics Eagle Health Supplies, Inc. Jascorp Pacific Pharmacy Computers Aetrex Worldwide, Inc. ECRS Kelli’s Gift Shop Suppliers Package Express Center, Inc. AIMSCO/Delta Hi-Tech, Inc. EISAI KeyCentrix Inc. Paddock Laboratories, Inc. Alpharma Eli Lilly & Company Kinray Inc. Pakor Inc. American Lifeline Emdeon Business Services Kirby Lester, LLC Parata Systems American Pharmacists Emporos Systems Lexi-Comp ParMed Pharmaceuticals Association Endo Pharmaceuticals Inc. Liberty Photo Products Partners in Pharmacy American Society of EPIC Pharmacies Inc. Life Line Screening PBA /TrueCare Pharmacies Consultant Pharmacists eRx Network, LLC Life-File LLC PDQ Communications Inc. AmerisourceBergen ETHEX Corporation LifeScan Inc. PDX-RX.com-PCI-FDS Corporation EXP Pharmaceutical Managed Health Care Pharmacist e-link Anda Inc. Services Corp. Associates Inc. Pharmacists Mutual Companies Apotex Corporation FDS, Inc. Mason Vitamins Inc. Pharmacists OnLine Apothecary Products Inc. Federation of Pharmacy Masters Pharmaceutical, a Pharmacy Choice, Inc. Apothecary Rx Networks Div. of DBS Trading Inc. Pharmacy Consulting Associates Associated Pharmacies, Inc. Fillmaster Systems LLC Maxim Staffing Solutions Pharmacy Development Services Astellas Pharma US Inc. First DataBank McKesson Pharmacy First/Wholesale AstraZeneca Flavorx McQueary Brothers Drug Co. Alliance LLC Ateb, Inc. G & W Laboratories, Inc. Meadowbrook Insurance Group Pharmacy Times Auburn Pharmaceutical G+M North America, Inc. Medical Matrix LP Pharmex, a Div of Time Auxilium Pharmaceuticals, Inc. Gallipot Inc. Medicine Shoppe Med Labeling Bayer Healthcare GeriMed/Rx Med/IV Med International, Inc. Pill Box, The Pharmaceuticals Gifts for Medical Professionals Medisca Inc. -
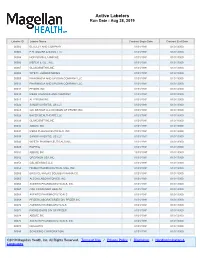
Active Labelers Run Date : Aug 28, 2019
Active Labelers Run Date : Aug 28, 2019 Labeler ID Labeler Name Contract Begin Date Contract End Date 00002 ELI LILLY AND COMPANY 01/01/1991 01/01/3000 00003 E.R. SQUIBB & SONS, LLC. 01/01/1991 01/01/3000 00004 HOFFMANN-LA ROCHE 01/01/1991 01/01/3000 00006 MERCK & CO., INC. 01/01/1991 01/01/3000 00007 GLAXOSMITHKLINE 01/01/1991 01/01/3000 00008 WYETH LABORATORIES 01/01/1991 01/01/3000 00009 PHARMACIA AND UPJOHN COMPANY LLC 01/01/1991 01/01/3000 00013 PHARMACIA AND UPJOHN COMPANY LLC 01/01/1991 01/01/3000 00014 PFIZER, INC 01/01/1991 01/01/3000 00015 MEAD JOHNSON AND COMPANY 01/01/1991 01/01/3000 00023 ALLERGAN INC 01/01/1991 01/01/3000 00024 SANOFI-AVENTIS, US LLC 01/01/1991 01/01/3000 00025 GD. SEARLE LLC DIVISION OF PFIZER INC. 01/01/1991 01/01/3000 00026 BAYER HEALTHCARE LLC 01/01/1991 01/01/3000 00029 GLAXOSMITHKLINE 01/01/1991 01/01/3000 00032 ABBVIE INC. 01/01/1991 01/01/3000 00037 MEDA PHARMACEUTICALS, INC. 01/01/1991 01/01/3000 00039 SANOFI-AVENTIS, US LLC 01/01/1991 01/01/3000 00046 WYETH PHARMACEUTICALS INC. 01/01/1991 01/01/3000 00049 ROERIG 01/01/1991 01/01/3000 00051 ABBVIE INC 10/01/1997 01/01/3000 00052 ORGANON USA INC. 01/01/1991 01/01/3000 00053 CSL BEHRING LLC 01/01/1991 01/01/3000 00054 HIKMA PHARMACEUTICAL USA, INC. 01/01/1991 01/01/3000 00056 BRISTOL-MYERS SQUIBB PHARMA CO. -

Appendix B - Product Name Sorted by Applicant
JUNE 2021 - APPROVED DRUG PRODUCT LIST B - 1 APPENDIX B - PRODUCT NAME SORTED BY APPLICANT ** 3 ** 3D IMAGING DRUG * 3D IMAGING DRUG DESIGN AND DEVELOPMENT LLC AMMONIA N 13, AMMONIA N-13 FLUDEOXYGLUCOSE F18, FLUDEOXYGLUCOSE F-18 SODIUM FLUORIDE F-18, SODIUM FLUORIDE F-18 3M * 3M CO PERIDEX, CHLORHEXIDINE GLUCONATE * 3M HEALTH CARE INC AVAGARD, ALCOHOL (OTC) DURAPREP, IODINE POVACRYLEX (OTC) 3M HEALTH CARE * 3M HEALTH CARE INFECTION PREVENTION DIV SOLUPREP, CHLORHEXIDINE GLUCONATE (OTC) ** 6 ** 60 DEGREES PHARMS * 60 DEGREES PHARMACEUTICALS LLC ARAKODA, TAFENOQUINE SUCCINATE ** A ** AAA USA INC * ADVANCED ACCELERATOR APPLICATIONS USA INC LUTATHERA, LUTETIUM DOTATATE LU-177 NETSPOT, GALLIUM DOTATATE GA-68 AAIPHARMA LLC * AAIPHARMA LLC AZASAN, AZATHIOPRINE ABBVIE * ABBVIE INC ANDROGEL, TESTOSTERONE CYCLOSPORINE, CYCLOSPORINE DEPAKOTE ER, DIVALPROEX SODIUM DEPAKOTE, DIVALPROEX SODIUM GENGRAF, CYCLOSPORINE K-TAB, POTASSIUM CHLORIDE KALETRA, LOPINAVIR NIASPAN, NIACIN NIMBEX PRESERVATIVE FREE, CISATRACURIUM BESYLATE NIMBEX, CISATRACURIUM BESYLATE NORVIR, RITONAVIR SYNTHROID, LEVOTHYROXINE SODIUM ** TARKA, TRANDOLAPRIL TRICOR, FENOFIBRATE TRILIPIX, CHOLINE FENOFIBRATE ULTANE, SEVOFLURANE ZEMPLAR, PARICALCITOL ABBVIE ENDOCRINE * ABBVIE ENDOCRINE INC LUPANETA PACK, LEUPROLIDE ACETATE ABBVIE ENDOCRINE INC * ABBVIE ENDOCRINE INC LUPRON DEPOT, LEUPROLIDE ACETATE LUPRON DEPOT-PED KIT, LEUPROLIDE ACETATE ABBVIE INC * ABBVIE INC DUOPA, CARBIDOPA MAVYRET, GLECAPREVIR NORVIR, RITONAVIR ORIAHNN (COPACKAGED), ELAGOLIX SODIUM,ESTRADIOL,NORETHINDRONE ACETATE -
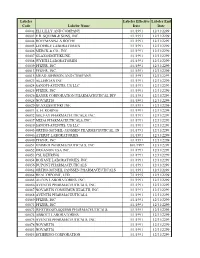
Participating Labelers.Xlsx
Labeler Labeler Effective Labeler End Code Labeler Name Date Date 00002 ELI LILLY AND COMPANY 1/1/1991 12/31/2299 00003 E.R. SQUIBB & SONS, INC. 1/1/1991 12/31/2299 00004 HOFFMANN-LA ROCHE 1/1/1991 12/31/2299 00005 LEDERLE LABORATORIES 1/1/1991 12/31/2299 00006 MERCK & CO., INC. 1/1/1991 12/31/2299 00007 GLAXOSMITHKLINE 1/1/1991 12/31/2299 00008 WYETH LABORATORIES 1/1/1991 12/31/2299 00009 PFIZER, INC 1/1/1991 12/31/2299 00013 PFIZER, INC. 1/1/1991 12/31/2299 00015 MEAD JOHNSON AND COMPANY 1/1/1991 12/31/2299 00023 ALLERGAN INC 1/1/1991 12/31/2299 00024 SANOFI-AVENTIS, US LLC 1/1/1991 12/31/2299 00025 PFIZER, INC. 1/1/1991 12/31/2299 00026 BAYER CORPORATION PHARMACEUTICAL DIV. 1/1/1991 12/31/2299 00028 NOVARTIS 1/1/1991 12/31/2299 00029 GLAXOSMITHKLINE 1/1/1991 12/31/2299 00031 A. H. ROBINS 1/1/1991 12/31/2299 00032 SOLVAY PHARMACEUTICALS, INC. 1/1/1991 12/31/2299 00037 MEDA PHARMACEUTICALS, INC. 1/1/1991 12/31/2299 00039 SANOFI-AVENTIS, US LLC 1/1/1991 12/31/2299 00045 ORTHO-MCNEIL-JANSSEN PHARMECEUTICAL, IN 1/1/1991 12/31/2299 00046 AYERST LABORATORIES 1/1/1991 12/31/2299 00049 PFIZER, INC 1/1/1991 12/31/2299 00051 UNIMED PHARMACEUTICALS, INC 10/1/1997 12/31/2299 00052 ORGANON USA INC. 1/1/1991 12/31/2299 00053 CSL BEHRING 1/1/1991 12/31/2299 00054 ROXANE LABORATORIES, INC. -

05/09/2016 Provider Subsystem Healthcare and Family Services Run Time: 04:25:50 Report Id 2794D051 Page: 01
MEDICAID SYSTEM (MMIS) ILLINOIS DEPARTMENT OF RUN DATE: 05/09/2016 PROVIDER SUBSYSTEM HEALTHCARE AND FAMILY SERVICES RUN TIME: 04:25:50 REPORT ID 2794D051 PAGE: 01 NUMERIC COMPLETE LIST OF PHARMACEUTICAL LABELERS WITH SIGNED REBATE AGREEMENTS IN EFFECT AS OF 07/01/2016 NDC NDC PREFIX LABELER NAME PREFIX LABELER NAME 00002 ELI LILLY AND COMPANY 00145 STIEFEL LABORATORIES, INC, 00003 E.R. SQUIBB & SONS, LLC. 00149 WARNER CHILCOTT PHARMACEUTICALS INC. 00004 HOFFMANN-LA ROCHE 00168 E FOUGERA AND CO. 00006 MERCK & CO., INC. 00169 NOVO NORDISK, INC. 00007 GLAXOSMITHKLINE 00172 IVAX PHARMACEUTICALS, INC. 00008 WYETH LABORATORIES 00173 GLAXOSMITHKLINE 00009 PFIZER, INC 00178 MISSION PHARMACAL COMPANY 00013 PFIZER, INC. 00182 GOLDLINE LABORATORIES, INC. 00015 MEAD JOHNSON AND COMPANY 00185 EON LABS, INC. 00023 ALLERGAN INC 00186 ASTRAZENECA LP 00024 SANOFI-AVENTIS, US LLC 00187 VALEANT PHARMACEUTICALS NORTH AMERICA 00025 PFIZER, INC. 00206 LEDERLE PIPERACILLIN 00026 BAYER HEALTHCARE LLC 00224 KONSYL PHARMACEUTICALS, INC. 00029 GLAXOSMITHKLINE 00225 B. F. ASCHER AND COMPANY, INC. 00032 SOLVAY PHARMACEUTICALS, INC. 00228 ACTAVIS ELIZABETH LLC 00037 MEDA PHARMACEUTICALS, INC. 00245 UPSHER-SMITH LABORATORIES, INC. 00039 SANOFI-AVENTIS, US LLC 00258 FOREST LABORATORIES INC 00046 AYERST LABORATORIES 00259 MERZ PHARMACEUTICALS 00049 PFIZER, INC 00264 B. BRAUN MEDICAL INC. 00051 UNIMED PHARMACEUTICALS, INC 00281 SAVAGE LABORATORIES 00052 ORGANON USA INC. 00299 GALDERMA LABORATORIES, L.P. 00053 CSL BEHRING 00300 TAP PHARMACEUTICALS INC 00054 ROXANE LABORATORIES, INC. 00310 ASTRAZENECA LP 00056 BRISTOL-MYERS SQUIBB PHARMA CO. 00327 GUARDIAN LABS DIV UNITED-GUARDIAN INC 00062 ORTHO MCNEIL PHARMACEUTICALS 00338 BAXTER HEALTHCARE CORPORATION 00064 HEALTHPOINT, LTD. 00378 MYLAN PHARMACEUTICALS, INC. -

~~-P~- Date Signed: _____
12151N ADDENDUM OF AGREEMENT THIS ADDENDUM, made and entered into effective as of the date by which both parties hereto have executed this document, by and between the County of Dane (hereinafter referred to as "County") and Premier Healthcare Alliance LP hereinafter, "Provider"). WITNESSETH: WHEREAS Provider and County, by a separate document (hereinafter, the "Master Agreement"), Purchase of Services Agreement No. 12151, have previously entered into a contractual relationship with Provider for the purpose of gaining access to Provider's group purchasing program for pharmaceuticals, medical equipment and supplies and related products and services to provide a means for County's Badger Prairie Health Care Center and other County departments or agencies to procure pharmaceuticals, medical equipment and supplies and related products and services at a reduced cost through group purchasing, and WHEREAS County and Provider wish to amend the Master Agreement in order to replace Appendix 1: List of Premier Suppliers as of 10/01/2019. NOW, THEREFORE, in consideration of the above premises and the mutual covenants of the parties hereinafter set forth, the receipt and sufficiency of which is hereby acknowledged by each party for itself, the parties do agree as follows: 1. The Master Agreement shall remain in full force and effect unchanged in any manner by this addendum except as changes are expressly set forth herein. This addendum shall control only to the extent of any conflict between the terms of the Master Agreement and this addendum. 2. Appendix 1: List of Premier Suppliers dated 07/01/2019 is replaced in full by Appendix 1: List of Premier Suppliers dated 10/01/2019. -

08/06/2016 Provider Subsystem Healthcare and Family Services Run Time: 00:10:55 Report Id 2794D051 Page: 01
MEDICAID SYSTEM (MMIS) ILLINOIS DEPARTMENT OF RUN DATE: 08/06/2016 PROVIDER SUBSYSTEM HEALTHCARE AND FAMILY SERVICES RUN TIME: 00:10:55 REPORT ID 2794D051 PAGE: 01 NUMERIC COMPLETE LIST OF PHARMACEUTICAL LABELERS WITH SIGNED REBATE AGREEMENTS IN EFFECT AS OF 10/01/2016 NDC NDC PREFIX LABELER NAME PREFIX LABELER NAME 00002 ELI LILLY AND COMPANY 00145 STIEFEL LABORATORIES, INC, 00003 E.R. SQUIBB & SONS, LLC. 00149 WARNER CHILCOTT PHARMACEUTICALS INC. 00004 HOFFMANN-LA ROCHE 00168 E FOUGERA AND CO. 00006 MERCK & CO., INC. 00169 NOVO NORDISK, INC. 00007 GLAXOSMITHKLINE 00172 IVAX PHARMACEUTICALS, INC. 00008 WYETH LABORATORIES 00173 GLAXOSMITHKLINE 00009 PFIZER, INC 00178 MISSION PHARMACAL COMPANY 00013 PFIZER, INC. 00182 GOLDLINE LABORATORIES, INC. 00015 MEAD JOHNSON AND COMPANY 00185 EON LABS, INC. 00023 ALLERGAN INC 00186 ASTRAZENECA LP 00024 SANOFI-AVENTIS, US LLC 00187 VALEANT PHARMACEUTICALS NORTH AMERICA 00025 PFIZER, INC. 00206 LEDERLE PIPERACILLIN 00026 BAYER HEALTHCARE LLC 00224 KONSYL PHARMACEUTICALS, INC. 00029 GLAXOSMITHKLINE 00225 B. F. ASCHER AND COMPANY, INC. 00032 SOLVAY PHARMACEUTICALS, INC. 00228 ACTAVIS ELIZABETH LLC 00037 MEDA PHARMACEUTICALS, INC. 00245 UPSHER-SMITH LABORATORIES, INC. 00039 SANOFI-AVENTIS, US LLC 00258 FOREST LABORATORIES INC 00046 AYERST LABORATORIES 00259 MERZ PHARMACEUTICALS 00049 PFIZER, INC 00264 B. BRAUN MEDICAL INC. 00051 UNIMED PHARMACEUTICALS, INC 00281 SAVAGE LABORATORIES 00052 ORGANON USA INC. 00299 GALDERMA LABORATORIES, L.P. 00053 CSL BEHRING 00300 TAP PHARMACEUTICALS INC 00054 ROXANE LABORATORIES, INC. 00310 ASTRAZENECA LP 00056 BRISTOL-MYERS SQUIBB PHARMA CO. 00327 GUARDIAN LABS DIV UNITED-GUARDIAN INC 00062 ORTHO MCNEIL PHARMACEUTICALS 00338 BAXTER HEALTHCARE CORPORATION 00064 HEALTHPOINT, LTD. 00378 MYLAN PHARMACEUTICALS, INC. -

Manufacturers with Signed Rebate Agreements
Wisconsin Medicaid Pharmacy Data Table Manufacturers with Signed Rebate Agreements January 1, 2013 NEWLABELER NAME START END SC NEW LABELER NAME START END SC 00002 ELI LILLY AND COMPANY 1/1/1991 Y 00131 KREMERS URBAN PHARMACEUTI 1/1/1991 Y 00003 E.R. SQUIBB & SONS, INC. 1/1/1991 Y 00132 C B FLEET COMPANY INC 1/1/1991 00004 HOFFMANN-LA ROCHE 1/1/1991 00135 GLAXOSMITHKLINE 1/1/1995 Y 00005 LEDERLE LABORATORIES 1/1/1991 Y 00143 WEST-WARD PHARMACEUTICAL C 1/1/1991 Y 00006 MERCK & CO., INC. 1/1/1991 Y 00145 STIEFEL LABORATORIES, INC, 1/1/1991 00007 GLAXOSMITHKLINE 1/1/1991 00149 PROCTER & GAMBLE PHARMACE 1/1/1991 00008 WYETH LABORATORIES 1/1/1991 Y 00168 E FOUGERA AND CO. 1/1/1991 Y 00009 PFIZER, INC 1/1/1991 Y 00169 NOVO NORDISK, INC. 1/1/1991 Y 00013 PFIZER, INC. 1/1/1991 Y 00172 IVAX PHARMACEUTICALS, INC. 1/1/1991 Y 00015 MEAD JOHNSON AND COMPANY 1/1/1991 Y 00173 GLAXOSMITHKLINE 1/1/1991 00023 ALLERGAN INC 1/1/1991 Y 00178 MISSION PHARMACAL COMPANY 1/1/1991 Y 00024 SANOFI-AVENTIS, US LLC 1/1/1991 Y 00182 GOLDLINE LABORATORIES, INC. 1/1/1991 Y 00025 PFIZER, INC. 1/1/1991 Y 00185 EON LABS, INC. 1/1/1991 Y 00026 BAYER CORPORATION PHARMAC 1/1/1991 Y 00186 ASTRAZENECA LP 1/1/1991 Y 00029 GLAXOSMITHKLINE 1/1/1991 00187 VALEANT PHARMACEUTICALS NO 1/1/1991 Y 00031 A. H. ROBINS 1/1/1991 Y 00206 LEDERLE PIPERACILLIN 1/1/1991 Y 00032 SOLVAY PHARMACEUTICALS, INC.