Pharma-Manufacturers.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

By in Vivo's Biopharma, Medtech and Diagnostics Teams
invivo.pharmaintelligence.informa.com JANUARY 2018 Invol. 36 ❚ no. 01 Vivopharma intelligence ❚ informa 2018 OUTLOOK By In Vivo’s Biopharma, Medtech and Diagnostics Teams PAGE LEFT BLANK INTENTIONALLY invivo.pharmaintelligence.informa.com STRATEGIC INSIGHTS FOR LIFE SCIENCES DECISION-MAKERS CONTENTS ❚ In Vivo Pharma intelligence | January 2018 BIOPHARMA MEDTECH 2018 DIAGNOSTICS OUTLOOK 12 22 28 Biopharma 2018: Medtech 2018: Diagnostics 2018: Is There Still A Place For Pharma The Place For Innovation Steady Progress And In The New Health Care As Value-based Health Care The Big Get Bigger Economy? Gains Momentum MARK RATNER WILLIAM LOONEY ASHLEY YEO If the beginning of 2017 was marked 2018 will be a time of transition in health 2017 was a watershed year in many by doubts around whether and how care, when biopharma’s counterparts respects, politically, economically the FDA would act with respect to in adjacent industry segments scale up and commercially for many players complex diagnostics, we enter 2018 in a radical redesign of their traditional in the medtech field. Where will the feeling that slow-moving vessel may business models. Biopharma is not opportunities lie in 2018? Will finally be turning. moving as quickly, and it confronts a breakthrough medtech innovation still strategic dilemma on how to address the have a place among providers often prospect of a much more powerful set of riding on fumes when it comes to 36 rivals in the ongoing battle to own the budgets, and is it all as bad as some patient experience in medicine. would make out? Thirty-five Years Covering Health Care: The More Things Change… 30 PETER CHARLISH A Virtuous Cycle: What The The health care industry has come a Immuno-Oncology Revolution long way in the past 35 years, although Means For Other Disease Areas in some areas very little has changed. -

In the United States District Court for the Eastern District of Pennsylvania ______: in Re Suboxone (Buprenorphine : Mdl No
IN THE UNITED STATES DISTRICT COURT FOR THE EASTERN DISTRICT OF PENNSYLVANIA __________________________________________ : IN RE SUBOXONE (BUPRENORPHINE : MDL NO. 2445 HYDROCHLORIDE AND NALOXONE) : 13-MD-2445 ANTITRUST LITIGATION : : THIS DOCUMENT RELATES TO:, : : Wisconsin, et al. v. Indivior Inc. et al. : Case No. 16-cv-5073 : __________________________________________: STATE OF WISCONSIN : By Attorney General Brad D. Schimel, et al. : : CIV. A. NO. 16-5073 Plaintiffs, : v. : : INDIVIOR INC. f/k/a RECKITT BENCKISER : PHARMACEUTICALS, INC., et al. : : Defendants. : __________________________________________: Goldberg, J. November 24, 2020 MEMORANDUM Defendant Reckitt Benckiser, Inc. (“Defendant”) manufactures Suboxone, a drug commonly used to combat opioid addiction.1 Suboxone previously came in tablet form, but in 2010, citing safety concerns, Defendant effectuated a change in the administration of this drug, switching from tablet to sublingual film. Various purchasers/consumers of Suboxone claimed that this switch was anticompetitive and solely designed to maintain Defendant’s market exclusivity—a scheme known as a “product hop.” These claims have resulted in multi-district, antitrust litigation before this Court. 1 Reckitt is currently known as Indivior, Inc. In December 2014, Reckitt Benckiser Pharmaceuticals, Inc. was demerged from its prior parent, the Reckitt Benckiser Group PLC, into Indivior PLC. Although Indivior is technically the named defendant in this case, the pleadings and many of the relevant exhibits use the name “Reckitt.” As discovery and class certification litigation have come to a close, the parties have raised numerous challenges under Daubert v. Merrell Dow Pharmaceuticals, 509 U.S. 579 (1993), seeking exclusion of all or selected portions of nine expert witnesses anticipated opinions. This Opinion explains my reasoning for the resolution of these motions and will hopefully set forth a clearer path towards trial. -

Transcept Pharmaceuticals, Inc
Table of Contents SCHEDULE 14A (RULE 14A 101) INFORMATION REQUIRED IN PROXY STATEMENT SCHEDULE 14A INFORMATION PROXY STATEMENT PURSUANT TO SECTION 14(A) OF THE SECURITIES EXCHANGE ACT OF 1934 (AMENDMENT NO. ) Filed by the Registrant x Filed by a Party other than the Registrant ¨ Check the appropriate box: ¨ Preliminary Proxy Statement ¨ Confidential, for Use of the Commission Only (as permitted by x Definitive Proxy Statement Rule 14a-6(e)(2)) ¨ Definitive Additional Materials ¨ Soliciting Material Pursuant to §240.14a-12 Transcept Pharmaceuticals, Inc. (Name of Registrant as Specified in its Charter) (Name of Person(s) Filing Proxy Statement, if other than the Registrant) Payment of Filing Fee (Check the appropriate box): x No fee required. ¨ Fee computed on table below per Exchange Act Rules 14a-6(i)(1) and 0-11. (1) Title of each class of securities to which transaction applies: (2) Aggregate number of securities to which transaction applies: (3) Per unit price or other underlying value of transaction computed pursuant to Exchange Act Rule 0-11 (set forth the amount on which the filing fee is calculated and state how it was determined): (4) Proposed maximum aggregate value of transaction: (5) Total fee paid: ¨ Fee paid previously with preliminary materials. ¨ Check box if any part of the fee is offset as provided by Exchange Act Rule 0-11(a)(2) and identify the filing for which the offsetting fee was paid previously. Identify the previous filing by registration statement number, or the Form or Schedule and the date of its filing. (1) Amount Previously Paid: (2) Form, Schedule or Registration Statement No.: (3) Filing Party: (4) Date Filed: Table of Contents NOTICE OF ANNUAL MEETING OF STOCKHOLDERS JUNE 23, 2011 To Our Stockholders: NOTICE IS HEREBY GIVEN that the Annual Meeting of Stockholders of Transcept Pharmaceuticals, Inc., a Delaware corporation, will be held on Thursday, June 23, 2011, at 8:30 a.m., local time, at our office located at 1003 West Cutting Blvd., Suite 110, Point Richmond, California 94804, for the following purposes: 1. -

Infectious Diseases
2013 MEDICINES IN DEVELOPMENT REPORT Infectious Diseases A Report on Diseases Caused by Bacteria, Viruses, Fungi and Parasites PRESENTED BY AMERICA’S BIOPHARMACEUTICAL RESEARCH COMPANIES Biopharmaceutical Research Evolves Against Infectious Diseases with Nearly 400 Medicines and Vaccines in Testing Throughout history, infectious diseases hepatitis C that inhibits the enzyme have taken a devastating toll on the lives essential for viral replication. and well-being of people around the • An anti-malarial drug that has shown Medicines in Development world. Caused when pathogens such activity against Plasmodium falci- For Infectious Diseases as bacteria or viruses enter a body and parum malaria which is resistant to multiply, infectious diseases were the current treatments. Application leading cause of death in the United Submitted States until the 1920s. Today, vaccines • A potential new antibiotic to treat methicillin-resistant Staphylococcus Phase III and infectious disease treatments have proven to be effective treatments in aureus (MRSA). Phase II many cases, but infectious diseases still • A novel treatment that works by Phase I pose a very serious threat to patients. blocking the ability of the smallpox Recently, some infectious pathogens, virus to spread to other cells, thus 226 such as pseudomonas bacteria, have preventing it from causing disease. become resistant to available treatments. Infectious diseases may never be fully Diseases once considered conquered, eradicated. However, new knowledge, such as tuberculosis, have reemerged new technologies, and the continuing as a growing health threat. commitment of America’s biopharma- America’s biopharmaceutical research ceutical research companies can help companies are developing 394 medicines meet the continuing—and ever-changing and vaccines to combat the many threats —threat from infectious diseases. -

Case Study of Nestlé1
CASE STUDY OF NESTLÉ1 INDEX PART A 1. Introduction 3 2. History 3 3. Industry Analysis and Competitors 5 3.1 Challenges of the food and beverage industry 6 3.2 Sales evolution of the industry 6 3.3 Qualitative Analysis: SWOT industry 8 3.4 Main competitors 9 3.5 Market Share 9 4. Business Model 10 4.1 Mission 10 4.2 Distinctive Factors 10 4.3 Corporate Governance 11 4.4 Corporate Social Responsibility 11 4.5 Segmentation of products 13 5. Questions 13 6. Bibliography 13 7. Notes 14 1 Case written by Clara Aguilar, Cristina Hey, Laura Plaza and Sara Zayas and supervised by Oriol Amat, BSM Universitat Pompeu Fabra, 2018 8. Annex 14 8.1 Balance Sheet 14 8.2 Income Statement 17 8.3 Cash Flow Statement 18 8.4 Ratios 20 PART B 1. Answer to the Questions Raised 22 2 PART A 1. INTRODUCTION “Nestlé” is a Swiss multinational food and beverage company whose business started in 1866. It is one of the largest food companies in the world, with presence in 191 countries, and more than 2,000 brands. Some of these are globally iconic while others are just regional, presenting a great variety of products, such as tea, coffee, bottled water, medical and baby food, breakfast cereals, and lots more. It is a well-known company world-wide, specially because of Nestlé milk chocolate bar, which is one of the most famous products. The company focuses on the production and supply of great quality and healthy food products. Nestle has a huge portfolio and is seen as an enormous competitor across the food industries. -

Global Competitiveness in Pharmaceuticals
Ref. Ares(2014)77485 - 15/01/2014 GLOBAL COMPETITIVENESS IN PHARMACEUTICALS A EUROPEAN PERSPECTIVE* * § ¨ ALFONSO GAMBARDELLA , LUIGI ORSENIGO , FABIO PAMMOLLI November 2000 Report prepared for the Enterprise Directorate-General of the European Commission * The authors wish to thank G. Baio, N. Lacetera, L. Magazzini, M. Mariani, R. Pammolli, and M. Riccaboni for skillfull research assistance. * Sant’Anna School of Advanced Studies, Pisa, [email protected]. § Bocconi University, Milan, [email protected]. ¨ Faculty of Economics Richard M. Goodwin, University of Siena, [email protected]. Enterprise Papers Enterprise Papers are a mix of policy papers, sector-specific studies, and a combination of both. Written by the staff of the Enterprise Directorate-General, or by experts working in association with them, they aim to raise awareness of enterprise policy issues and stimulate debate. These papers do not necessarily reflect the opinion or position of the European Commission. Occasional ‘special editions’ may carry communications, conference proceedings, and reports to the Council. This report was prepared for the Enterprise Directorate-General by Alfonso Gambardella, Luigi Orsenigo and Fabio Pammolli. For further information, contact European Commission Enterprise Directorate-General Information and communication unit Rue de la Loi/ Wetstraat 200 B-1049 Brussels Fax: (32-2) 299 1926 To request copies, fax (32-2) 296 9930. E-mail: [email protected] A great deal of additional information on the European Union is available on the internet. It can be accessed through the Europa server (http://europa.eu.int). Luxembourg: Office for Official Publications of the European Communities, 2001 ISBN 92-894-1071-X © European Communities, 2001 Reproduction is authorised provided the source is acknowledged. -

Ethnic Migrant Media Forum 2014 | Curated Proceedings 1 FOREWORD
Ethnic Migrant Media Forum 2014 CURATED PROCEEDINGS “Are we reaching all New Zealanders?” Exploring the Role, Benefits, Challenges & Potential of Ethnic Media in New Zealand Edited by Evangelia Papoutsaki & Elena Kolesova with Laura Stephenson Ethnic Migrant Media Forum 2014. Curated Proceedings is licensed under a Creative Commons Attribution- NonCommercial 4.0 International License. Ethnic Migrant Media Forum, Unitec Institute of Technology Thursday 13 November, 8.45am–5.45pm Unitec Marae, Carrington Road, Mt Albert Auckland, New Zealand The Introduction and Discussion sections were blind peer-reviewed by a minimum of two referees. The content of this publication comprises mostly the proceedings of a publicly held forum. They reflect the participants’ opinions, and their inclusion in this publication does not necessarily constitute endorsement by the editors, ePress or Unitec Institute of Technology. This publication may be cited as: Papoutsaki, E. & Kolesova, E. (Eds.) (2017). Ethnic migrant media forum 2014. Curated proceedings. Auckland, New Zealand. Retrieved from http://unitec. ac.nz/epress/ Cover design by Louise Saunders Curated proceedings design and editing by ePress Editors: Evangelia Papoutsaki and Elena Kolesova with Laura Stephenson Photographers: Munawwar Naqvi and Ching-Ting Fu Contact [email protected] www.unitec.ac.nz/epress Unitec Institute of Technology Private Bag 92025, Victoria Street West Auckland 1142 New Zealand ISBN 978-1-927214-20-6 Marcus Williams, Dean of Research and Enterprise (Unitec) opens the forum -

“Analysis of SCM: a Case Study of Wyeth Pharmaceutical Company”
“Analysis of SCM: A case study of Wyeth Pharmaceutical company” Submitted by: Shahzad Ali Rajpar Supervised by: Mr. Muhammad Amir Adam Program: MBA FALL 2010 National University of Computer & Emerging Science Management Science Department, Karachi, Campus Page | 1 Acknowledgments Thanks to Allah the All Merciful the all Benevolent for providing me the strength, courage, direction and skills to learn, acquire knowledge, and the ability to accept and meet challenges. Second I would like to thank all those people who have helped me in performing this research study, especially Mr. Aftab Amie Siddiqui manager planning and warehouse, Wyeth Pharmaceutical Limited Pakistan. I would like to express my sincere gratitude to my supervisor Mr. Mohammad Amir Adam for providing me his precious time, guidance, and instructions all along in order to materialize my content for the project report. I would also like to thank the FYP Coordinator Mr. Zaki Rashidi for his assistance and guidance for the research project. I am also thankful to my parents who accommodated me during those long hours of work in my project development and all the friends and colleagues who helped me out in my times of weakness and encouraged me. I am hopeful that the effort will be fruitful for the students to come in FAST after us. Once again, I am very thankful to all people who have been involved in this project report directly or indirectly. Page | 2 Table of Contents Acknowledgments ............................................................................................. 2 Table of Contents .............................................................................................. 3 CHAPTER 1 “INTRODUCTION” ........................................................................... 6 1.1 Supply chain management. ..................................................................... 6 1.2 Evolution of supply chain management. -

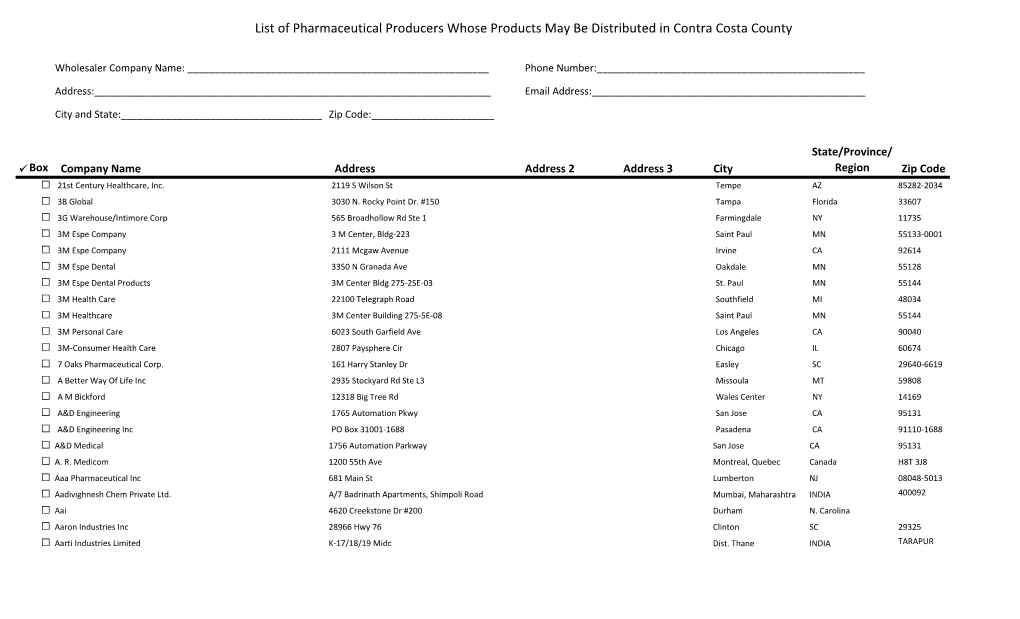
Manufacturers and Wholesalers Street
Nevada AB128 Code of Conduct Compliant Companies Manufacturers and Wholesalers Street City ST Zip 10 Edison Street LLC 13 Edison Street LLC Abbott Diabetes Care Division Abbott Diagnostic Division Abbott Electrophysiology (including Kalila Medical 2- 2016)) Abbott Laboratories 100 Abbott Park Road, Dept. EC10, Bldg. APGA-2 Abbott Park IL 60064 Abbott Medical Optics Abbott Molecular Division Abbott Nutrition Products Division Abbott Vascular Division (includes Tendyne 9-2015) AbbVie, Inc. 1 N. Waukegan Road North Chicago IL 60064 Acadia Phamaceuticals 3611 Valley Centre Drive, Suite 300 San Diego CA 92130 Accelero Health Partners, LLC Acclarent, Inc. 1525-B O'Brien Dr. Menlo Park CA 94025 Accuri Cyometers, Inc. Ace Surgical Supply, Inc. 1034 Pearl St. Brockton MA 02301 Acorda Therapeutics, Inc. 420 Sawmill River Road Ardsley NY 10532 AcriVet, Inc. Actavis W.C. Holding, Inc. Morris Corporate Center III, 400 Interpace Parkway Parsippany NJ 07054 Actavis , Inc. Actelion Pharmaceuticals US, Inc. 5000 Shoreline Court, Suite 200 S. San Francisco CA 94080 Activis 400 Interpace parkway Parsippany NJ 07054 A-Dec, Inc. 2601 Crestview Dr. Newberg OR 97132 Advanced Respiratory, Inc. Advanced Sterilization Products 33 Technology Drive Irvine CA 92618 Advanced Vision Research, Inc., dba Akorn Consumer Health Aegerion Pharmaceuticals, Inc. 101 Main Street, Suite 1850 Cambridge MA 02142 Aesculap Implant Systems, Inc. Aesculap, Inc. 3773 Corporate Parkway Center Valley PA 18034 Aesthera Corporation Afaxys, Inc. PO Box 20158 Charleston SC 29413 AGMS, Inc. Akorn (New Jersey) Inc. Page 1 of 23 Pages 2/15/2017 Nevada AB128 Code of Conduct Compliant Companies Akorn AG (formerly Excelvision AG) Akorn Animal Health, Inc. -

PDF-Xchange 4.0 Examples
WorldReginfo - f65a79fa-dec3-4614-8df6-74077a403cfa - WorldReginfo Annual Review 2015 Nestlé – Annual Review 2015 Our business Nestlé has grown from a company founded 150 years ago to a global leader in Nutrition, Health and Wellness. Wherever you are in What we sell (in CHF billion) the world we have safe, nutritious products to Powdered and Nutrition and Milk products Prepared dishes Liquid Beverages Health Science and Ice cream and cooking aids help you care for yourself and your family. Our product portfolio has seven categories, offering you 19.2 14.9 14.6 12.6 healthier and tastier choices at every stage of your life, at every time of the day. PetCare Confectionery Water 11.5 8.9 7.1 Our growth has enabled Where we sell (in CHF billion) us to help improve the lives of millions of people through the products EMENA and services we provide, 27.5 and through employment, our supplier networks and the contribution we make to economies around the world. AMS AOA 39.1 22.2 Number of employees Number of countries we sell in 335 000 189 Total group salaries and social Corporate taxes paid in 2015 welfare expenses (in CHF) (in CHF) 16 billion 3.3 billion WorldReginfo - f65a79fa-dec3-4614-8df6-74077a403cfa Our commitments Our 39 commitments in the Nestlé in society report guide all of us at Nestlé in our collective efforts to meet specific objectives. For a company to prosper Nutrition, health and wellness over the long term and create value for shareholders, it 192 billion 8041 must create value for society at the same time. -

The Impact of Secondary Innovation on Firm Market Value in the Pharmaceutical Industry
The Impact of Secondary Innovation on Firm Market Value in the Pharmaceutical Industry By: Maitri Punjabi Honors Thesis Economics Department The University of North Carolina at Chapel Hill March 2016 Approved: ______________________________ Dr. Jonathan Williams Punjabi 2 Abstract This paper analyzes the effect of the changing nature of innovation on pharmaceutical firm market value from the years 1987 to 2010 by using U.S. patent and claim data. Over the years, firms have started shifting focus from primary innovation to secondary innovation as new ideas and new compounds become more difficult to generate. In this study, we analyze the impact of this patent portfolio shift on the market capitalization of pharmaceutical firms. After using firm fixed effects and the instrumental variable approach, we find that there exists a strong positive relationship between secondary innovations and the market value of the firm– in fact, we find a stronger relationship than is observed between primary innovation and market value. When focusing on the different levels of innovation within the industry, we find that this relationship is stronger for less-innovative firms (those that have produced fewer patents) than it is for highly- innovative firms. We also find that this relationship is stronger for firms that spend less on research and development, complementing earlier findings that research productivity is declining over time. Punjabi 3 Acknowledgements I would primarily like to thank my adviser, Dr. Jonathan Williams, for his patience and constant support. Without his kind and helpful attitude, this project would have been a much more frustrating process. Through his knowledge of the industry, I have gained valuable insight and have learned a great deal about a unique and growing field. -

Preven Ng Pharmaceu Cal Pollu on and Diversion
Preven&ng Pharmaceu&cal Pollu&on and Diversion Kate Hagemann & Sierra Fletcher Product Stewardship Ins&tute How to Participate Today • Open and close your Panel • View, Select, and Test your audio • Submit text questions • Raise your hand • Q&A addressed at the end of today’s session • Everyone will receive an email within 24 hours with a link to view a recorded version of today’s session Who is the Product Stewardship Instute? § Non-profit founded in 2000 § Membership ü 47 States ü 200+ Local governments ü 70+ Corporate, Organizaonal, Academic & Non-U.S. Government Partners § Board of Directors: 7 states, 4 local agencies • Mul4-stakeholder product stewardship network 3 The Problem: Waste Pharmaceucals 1. Environmental Concerns 2. Drug Diversion concerns 3. Public Safety Concerns 4 1. Environmental Concerns • Effects in the environment: – Endocrine disruptors – An&bio&c resistance • Pharmaceu&cals enter the environment via a number of channels – Agricultural run-off – Human excre&on – Improper disposal • Current waste water treatment plants cannot remove pharmaceu&cal compounds April 15, 2011 5 Evidence of pharmaceucals In our waterways • Minnesota Pollu&on Control Agency (2011) • USGS (June 2002) •“a broad range of chemicals found in residen3al, industrial, and agricultural wastewaters commonly occurs in mixtures at low concentra3ons downstream from areas of intense urbaniza3on and animal producon. The chemicals include human and veterinary drugs (including an3bio3cs), natural and synthe3c hormones, detergent metabolites, plas3cizers, inseccides, and fire retardants. One or more of these chemicals were found in 80 percent of the streams sampled” April 15, 2011 6 Environmental Impacts • Ecological impacts remain unknown • Observed impacts: – Abnormali&es – Disrupts reproduc&ve systems/risk of ex&ncon • Baylor University researchers found residues human medicaons in fish.