Phyllocrania Paradoxa): Are Components of the Strike Stereotypic? Christopher E
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
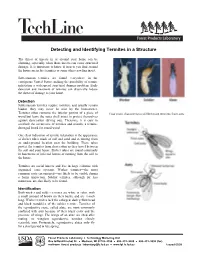
Termites in a Structure
Detecting and Identifying Termites in a Structure The threat of insects in or around your home can be alarming, especially when those insects can cause structural damage. It is important to know if insects you find around the house are in fact termites or some other crawling insect. Subterranean termites are found everywhere in the contiguous United States, making the possibility of termite infestation a widespread structural damage problem. Early detection and treatment of termites can drastically reduce the threat of damage to your home. Detection Subterranean termites require moisture and usually remain hidden—they may never be seen by the homeowner. Termites often consume the interior portion of a piece of Four main characteristics differentiate termites from ants: wood but leave the outer shell intact to protect themselves against desiccation (drying out). Therefore, it is easy to overlook the occurrence of termites and mistake a termite- damaged board for sound wood. One clear indication of termite infestation is the appearance of shelter tubes made of soil and sand and stemming from an underground location near the building. These tubes protect the termites from desiccation as they travel between the soil and your house. Shelter tubes are found commonly in basements of infected homes or running from the soil to the house. Termites are social insects and live in large colonies with organized caste systems. Worker termites—the most common caste encountered—are likely to be visible during a home inspection. Soldier termites, although far less numerous, are also likely to be found. Identification Both worker and soldier termites are white in color, with a small amount of brown on their backs, and are ¼-inch long. -

Mantodea (Insecta), with a Review of Aspects of Functional Morphology and Biology
aua o ew eaa Ramsay, G. W. 1990: Mantodea (Insecta), with a review of aspects of functional morphology and biology. Fauna of New Zealand 19, 96 pp. Editorial Advisory Group (aoimes mae o a oaioa asis MEMBERS AT DSIR PLANT PROTECTION Mou Ae eseac Cee iae ag Aucka ew eaa Ex officio ieco — M ogwo eae Sysemaics Gou — M S ugae Co-opted from within Systematics Group Dr B. A ooway Κ Cosy UIESIIES EESEAIE R. M. Emeso Eomoogy eame ico Uiesiy Caeuy ew eaa MUSEUMS EESEAIE M R. L. ama aua isoy Ui aioa Museum o iae ag Weigo ew eaa OESEAS REPRESENTATIVE J. F. awece CSIO iisio o Eomoogy GO o 1700, Caea Ciy AC 2601, Ausaia Series Editor M C ua Sysemaics Gou SI a oecio Mou Ae eseac Cee iae ag Aucka ew eaa aua o ew eaa Number 19 Maoea (Iseca wi a eiew o asecs o ucioa mooogy a ioogy G W Ramsay SI a oecio M Ae eseac Cee iae ag Aucka ew eaa emoa us wig mooogy eosigma cooaio siuaio acousic sesiiiy eece eaiou egeeaio eaio aasiism aoogy a ie Caaoguig-i-uicaio ciaio AMSAY GW Maoea (Iseca – Weigo SI uisig 199 (aua o ew eaa ISS 111-533 ; o 19 IS -77-51-1 I ie II Seies UC 59575(931 Date of publication: see cover of subsequent numbers Suggese om o ciaio amsay GW 199 Maoea (Iseca wi a eiew o asecs o ucioa mooogy a ioogy Fauna of New Zealand [no.] 19. —— Fauna o New Zealand is eae o uicaio y e Seies Eio usig comue- ase e ocessig ayou a ase ie ecoogy e Eioia Aisoy Gou a e Seies Eio ackowege e oowig co-oeaio SI UISIG awco – sueisio o oucio a isiuio M C Maews – assisace wi oucio a makeig Ms A Wig – assisace wi uiciy a isiuio MOU AE ESEAC CEE SI Miss M oy -

Norsk Lovtidend
Nr. 7 Side 1067–1285 NORSK LOVTIDEND Avd. I Lover og sentrale forskrifter mv. Nr. 7 Utgitt 30. juli 2015 Innhold Side Lover og ikrafttredelser. Delegering av myndighet 2015 Juni 19. Ikrafts. av lov 19. juni 2015 nr. 60 om endringer i helsepersonelloven og helsetilsynsloven (spesialistutdanningen m.m.) (Nr. 674) ................................................................1079................................ Juni 19. Ikrafts. av lov 19. juni 2015 nr. 77 om endringar i lov om Enhetsregisteret m.m. (registrering av sameigarar m.m.) (Nr. 675) ................................................................................................1079 ..................... Juni 19. Deleg. av Kongens myndighet til Helse- og omsorgsdepartementet for fastsettelse av forskrift for å gi helselover og -forskrifter hel eller delvis anvendelse på Svalbard og Jan Mayen (Nr. 676) ................................................................................................................................1080............................... Juni 19. Ikrafts. av lov 19. juni 2015 nr. 59 om endringer i helsepersonelloven mv. (vilkår for autorisasjon) (Nr. 678) ................................................................................................................................1084 ..................... Juni 19. Ikrafts. av lov 13. mars 2015 nr. 12 om endringer i stiftelsesloven (stiftelsesklagenemnd) (Nr. 679) ................................................................................................................................................................1084 -

The Genus Metallyticus Reviewed (Insecta: Mantodea)
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/228623877 The genus Metallyticus reviewed (Insecta: Mantodea) Article · September 2008 CITATIONS READS 11 353 1 author: Frank Wieland Pfalzmuseum für Naturkunde - POLLICHIA-… 33 PUBLICATIONS 113 CITATIONS SEE PROFILE All in-text references underlined in blue are linked to publications on ResearchGate, Available from: Frank Wieland letting you access and read them immediately. Retrieved on: 24 October 2016 Species, Phylogeny and Evolution 1, 3 (30.9.2008): 147-170. The genus Metallyticus reviewed (Insecta: Mantodea) Frank Wieland Johann-Friedrich-Blumenbach-Institut für Zoologie & Anthropologie und Zoologisches Museum der Georg-August-Universität, Abteilung für Morphologie, Systematik und Evolutionsbiologie, Berliner Str. 28, 37073 Göttingen, Germany [[email protected]] Abstract Metallyticus Westwood, 1835 (Insecta: Dictyoptera: Mantodea) is one of the most fascinating praying mantids but little is known of its biology. Several morphological traits are plesiomorphic, such as the short prothorax, characters of the wing venation and possibly also the lack of discoidal spines on the fore femora. On the other hand, Metallyticus has autapomor- phies which are unique among extant Mantodea, such as the iridescent bluish-green body coloration and the enlargement of the first posteroventral spine of the fore femora. The present publication reviews our knowledge of Metallyticus thus providing a basis for further research. Data on 115 Metallyticus specimens are gathered and interpreted. The Latin original descriptions of the five Metallyticus species known to date, as well as additional descriptions and a key to species level that were originally published by Giglio-Tos (1927) in French, are translated into English. -
MSG NL 27 (October 2007).Pdf
ISSN 1364-3193 Mantis Study Group Newsletter 27 October 2007 Editorial – P.E. Bragg. Welcome to the final MSG Newsletter – at least the final one of this series. The previous Newsletter, volume 26, was issued in August 2003. No more articles were received for publication after that date and with no Newsletters being produced, the Mantis Study Group effectively ceased to exist. In producing this Newsletter, I am not attempting a revival: the opposite is closer to the truth. This issue is intended to update a few previously published items, and to bring the publication to a conclusion by issuing an index to all the previous Newsletters. My thanks go to the 48 contributors to the Newsletters. There is a larger interest in mantids than ever before, judging by the numbers sold at exhibitions in the UK. A much wider range of species is available than when the MSG started in 1996. Over the past four years several people have raised the possibility of re-launching the MSG. A common theme in the suggestions has been to use the internet as a platform. That is rather ironic because, to some extent, the spread of the internet and availability of information on the net was responsible for the demise of the Newsletter; as information became more readily available there was less incentive for people to join the MSG, and less incentive for people to write articles for the Newsletter. The cost of printing and postage means that any revitalisation of the MSG will undoubtedly be internet based. The Newsletter was always short of illustrations, because it could only accommodate black and white drawings, but the internet, coupled with easy access to digital cameras, means that colour photographs can be issued, effectively at zero cost. -

03 Literature Review
- Literature Review: - Sr. No .1 - Name of the Author : P.G. Koehler and C. L. Tucker - Name of the Article : Subterranean Termite - Pages: 7 Nos. - Date of Published : April 1993 Revised in 2003 University of Florida, IFAS, Extention No. ENY 210 - Journal Name : Entomology and Nematology, Florida Cooperative Service - Review: Subterranean Termite, Dampwood and Drywood Termite are the main three types of termites . Subterranean Termites are the most destructive and it attacks structures by building tubes from nest to wood structures. They feed on wood which contain cellulose which is digested by them by protozoa and convert cellulose into usable food. The nest in the soil contains moisture to protect them from low humidity and predation and they build mud tunnels or tubes to reach wood. At swarming stage the broken wings are located at light are hence they are attracted to light. No wood contact with soil, ventilation, regular inspection can avoid the entry of termite and for effective control for Subterranean termite, Pre and post treatment methods for new and for existing structures at different stages with repellent and non repellent insecticides as required with the additional control method of using Termite Bait. - Sr. No . 2 - Name of the Author : Matthew R. Tarver, Christopher B. Florane, Dunhua Zhang, Casey Grimm and Alan R. Lax - Name of the Article : Methoprene and temperature effects on caste differentiation and protein composition in the Formosan Subterranean termite, Coptotermesfomosanus - Pages: 10 Nos. - Date of Published : December 2011 - Journal Name : Journal of Insect Science, Vol. 12/ article 18 - Review: Worker to soldier differentiation is modulated by temperature i.e. -

The Complete Mitochondrial Genome of Psychomantis Borneensis (Mantodea: Hymenopodidae)
Mitochondrial DNA Part B Resources ISSN: (Print) 2380-2359 (Online) Journal homepage: http://www.tandfonline.com/loi/tmdn20 The complete mitochondrial genome of Psychomantis borneensis (Mantodea: Hymenopodidae) Le-Ping Zhang, Yin-Yin Cai, Dan-Na Yu, Kenneth B. Storey & Jia-Yong Zhang To cite this article: Le-Ping Zhang, Yin-Yin Cai, Dan-Na Yu, Kenneth B. Storey & Jia-Yong Zhang (2018) The complete mitochondrial genome of Psychomantis borneensis (Mantodea: Hymenopodidae), Mitochondrial DNA Part B, 3:1, 42-43, DOI: 10.1080/23802359.2017.1419094 To link to this article: https://doi.org/10.1080/23802359.2017.1419094 © 2017 The Author(s). Published by Informa UK Limited, trading as Taylor & Francis Group. Published online: 21 Dec 2017. Submit your article to this journal Article views: 12 View related articles View Crossmark data Full Terms & Conditions of access and use can be found at http://www.tandfonline.com/action/journalInformation?journalCode=tmdn20 Download by: [134.117.97.124] Date: 08 January 2018, At: 06:28 MITOCHONDRIAL DNA PART B: RESOURCES, 2018 VOL. 3, NO. 1, 42–43 https://doi.org/10.1080/23802359.2017.1419094 MITOGENOME ANNOUNCEMENT The complete mitochondrial genome of Psychomantis borneensis (Mantodea: Hymenopodidae) Le-Ping Zhanga, Yin-Yin Caia, Dan-Na Yua,b, Kenneth B. Storeyc and Jia-Yong Zhanga,b,c aCollege of Chemistry and Life Science, Zhejiang Normal University, Jinhua, Zhejiang Province, China; bKey Lab of Wildlife Biotechnology, Conservation and Utilization of Zhejiang Province, Zhejiang Normal University, Jinhua, Zhejiang Province, China; cDepartment of Biology, Carleton University, Ottawa, Canada ABSTRACT ARTICLE HISTORY The complete mitochondrial genome of Psychomantis borneensis (Mantodea: Hymenopodidae) was suc- Received 8 December 2017 cessfully sequenced. -

Cryptic Female Mantids Signal Quality Through Brightness
Functional Ecology 2015, 29, 531–539 doi: 10.1111/1365-2435.12363 Sexual signals for the colour-blind: cryptic female mantids signal quality through brightness Katherine L. Barry*, Thomas E. White, Darshana N. Rathnayake, Scott A. Fabricant and Marie E. Herberstein Department of Biological Sciences, Macquarie University, Sydney, NSW 2109, Australia Summary 1. Cryptic coloration may evolve in response to selective pressure imposed by predators, yet effective intraspecific communication may require some level of detectability. This creates a tension between the benefits of sexually selected visual traits and the predatory costs imposed by greater conspicuousness, and little is known about how this tension may be ameliorated in highly cryptic species. 2. We explore these competing demands in the false garden mantid Pseudomantis albofimbriata, a colour-blind and seemingly cryptic insect. We use reflectance spectrometry and receptor-noise modelling to characterize the conspicuousness of mantid body regions in the visual systems of mates (mantids), as well as potential predators (birds) and prey (bees). We then use condition manipulation and conspecific choice tests to further explore the colour traits of interest. 3. Based on visual modelling, we find that male mantids are inconspicuous to conspecifics, prey and predators – that is, they are chromatically and achromatically cryptic. In contrast, female mantids are chromatically cryptic to all potential receivers, but their abdomens are achromatically conspicuous. Our food manipulation experiment shows that females in good condition (and therefore with more eggs) have brighter abdomens than females in poor condition. Choice assays show male mantids are consistently attracted to females bearing brighter abdomens. 4. Our results reveal brightness-mediated sexual signalling in a colour-blind and classically cryptic insect. -

Survey of the Superorder Sanghar, Sin Superorder
UNIVERSITY OF SINDH JOURNAL OF ANIMAL SCIENCES Vol. 2, Issue 1, pp: (19-23), April, 2018 Email: [email protected] ISSN(E) : 252 3-6067 Website: http://sujo.usindh.edu.pk/index.php/USJAS ISSN(P) : 2521-8328 © Published by University of Sindh, Jamshoro SURVEY OF THE SUPERORDER DICTYOPTERA MANTODEA FROM SANGHAR, SINDH , PAKISTAN Sadaf Fatimah, Riffat Sultana and Muhammad Saeed Wagan Department of Zoology, University of Sindh, Jamshoro ARTICLE INFORMATION ABSTRACT Article History: This paper deals with the fauna of different species of Mantodea from Received: 20 th December, 2017 Accepted: 10th April, 2018 different localities of Sanghar (district). A tota l of 16 species from 12 Published online: 15 th May, 2018 genera including (Blepharopsis, Empusa, Humbertiella, Deiphobe, Authors Contribution Archimantis, Hierodula, Mantis, Stalilia, Polyspilota, Iris , Rivetina and S.F collected the material and analyzed Eremphilia belong to 05 families Mantidae, Tarachodidae, Empusidae, the samples, R.S planned the study, and liturgusidae and Eremiaphilidae) were identified and presented. A M.S.W identified the material and finalized the result. All the authors read comparison of Pakistani Mantodea’s fauna at global level was also done and approved the final version of the and six new regional record species were also found and presented here. article. During this study significant numbers were captured. It was also noticed Key words: that its predatory behavior has very important for reorganization of it’s as Mantodea, Sanghar, bio-control agent. Empusidae, Liturgusidae, Mantidae, Tarachodidae . 1. INTRODUCTION ery rare papers published on praying mantids of 2. MATERIAL AND METHODS V(Sindh) Pakistan. -

Developing Biodiverse Green Roofs for Japan: Arthropod and Colonizer Plant Diversity on Harappa and Biotope Roofs
20182018 Green RoofsUrban and Naturalist Urban Biodiversity SpecialSpecial Issue No. Issue 1:16–38 No. 1 A. Nagase, Y. Yamada, T. Aoki, and M. Nomura URBAN NATURALIST Developing Biodiverse Green Roofs for Japan: Arthropod and Colonizer Plant Diversity on Harappa and Biotope Roofs Ayako Nagase1,*, Yoriyuki Yamada2, Tadataka Aoki2, and Masashi Nomura3 Abstract - Urban biodiversity is an important ecological goal that drives green-roof in- stallation. We studied 2 kinds of green roofs designed to optimize biodiversity benefits: the Harappa (extensive) roof and the Biotope (intensive) roof. The Harappa roof mimics vacant-lot vegetation. It is relatively inexpensive, is made from recycled materials, and features community participation in the processes of design, construction, and mainte- nance. The Biotope roof includes mainly native and host plant species for arthropods, as well as water features and stones to create a wide range of habitats. This study is the first to showcase the Harappa roof and to compare biodiversity on Harappa and Biotope roofs. Arthropod species richness was significantly greater on the Biotope roof. The Harappa roof had dynamic seasonal changes in vegetation and mainly provided habitats for grassland fauna. In contrast, the Biotope roof provided stable habitats for various arthropods. Herein, we outline a set of testable hypotheses for future comparison of these different types of green roofs aimed at supporting urban biodiversity. Introduction Rapid urban growth and associated anthropogenic environmental change have been identified as major threats to biodiversity at a global scale (Grimm et al. 2008, Güneralp and Seto 2013). Green roofs can partially compensate for the loss of green areas by replacing impervious rooftop surfaces and thus, contribute to urban biodiversity (Brenneisen 2006). -

Research in Biological Control of the Formosan Subterranean Termite Cai Wang Louisiana State University and Agricultural and Mechanical College, [email protected]
Louisiana State University LSU Digital Commons LSU Doctoral Dissertations Graduate School 2014 Research in Biological Control of the Formosan Subterranean Termite Cai Wang Louisiana State University and Agricultural and Mechanical College, [email protected] Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_dissertations Part of the Entomology Commons Recommended Citation Wang, Cai, "Research in Biological Control of the Formosan Subterranean Termite" (2014). LSU Doctoral Dissertations. 598. https://digitalcommons.lsu.edu/gradschool_dissertations/598 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Doctoral Dissertations by an authorized graduate school editor of LSU Digital Commons. For more information, please [email protected]. RESEARCH IN BIOLOGICAL CONTROL OF THE FORMOSAN SUBTERRANEAN TERMITE A Dissertation Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Doctor of Philosophy in The Department of Entomology by Cai Wang M.S., Chinese Academy of Science, 2010 B.S., Huazhong University of Science and Technology, 2007 August 2014 ACKNOWLEDGMENTS I would like to express my sincerest appreciation to my major professor, Dr. Gregg Henderson, a very important person in my life. I am very impressive for his meticulous attitude for scientific research. I benefited greatly from his valuable and illuminating suggestions for my research. It is also very touching for Dr. Henderson’s patience for my preliminary and sometimes “crazy” ideas. Also, he always could “see” what I ignored. For example, when I unintentionally talked about an observation that soldier and worker termites run in different directions after disturbance, he immediately pointed out the potential value to continue studying this and gave me valuable suggestions in the experiment. -

Fortschritte Und Perspektiven in Der Erforschung Der Evolution Und Phylogenie Der Mantodea (Insecta: Dictyoptera)
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Entomologie heute Jahr/Year: 2017 Band/Volume: 29 Autor(en)/Author(s): Wieland Frank, Schütte Kai Artikel/Article: Fortschritte und Perspektiven in der Erforschung der Evolution und Phylogenie der Mantodea (Insecta: Dictyoptera). Progress and Perspectives in Research on the Evolution and Phylogeny of Mantodea (Insecta: Dictyoptera) 1-23 Fortschritte und Perspektiven in der Erforschung der Mantodea 1 Entomologie heute 29 (2017): 1-23 Fortschritte und Perspektiven in der Erforschung der Evolution und Phylogenie der Mantodea (Insecta: Dictyoptera) Progress and Perspectives in Research on the Evolution and Phylogeny of Mantodea (Insecta: Dictyoptera) FRANK WIELAND & KAI SCHÜTTE Zusammenfassung: Die Gottesanbeterinnen (Mantodea) sind eine Insektengruppe, die vielen auch nicht naturkundlich interessierten Menschen bekannt ist – nicht zuletzt wegen des häufi g beobachteten Sexualkannibalismus. Das fremdartige und formenreiche Erscheinungsbild der 2.500 beschriebenen Arten hat auch die Entomologinnen und Entomologen seit jeher fasziniert. Die Erforschung der Gottesanbeterinnen entwickelte sich in den vergangenen Jahrhunderten jedoch eher gemächlich. Als morphologische und molekulare Analysen in den 1990er Jahren erstmals einen phylogenetisch-systematischen Ansatz verfolgten, begann eine neue Ära der Mantodea-Forschung. Der vorliegende Beitrag fasst die bedeutendsten Arbeiten zur Untersuchung zur phylogenetischen