Classification of Large Circulating Tumor Cells Isolated with Ultra- High Throughput Microfluidic Vortex Technology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Circulating Tumor Cell As a Diagnostic Marker in Primary Lung Cancer
Published OnlineFirst November 3, 2009; DOI: 10.1158/1078-0432.CCR-09-1095 Published Online First on November 3, 2009 as 10.1158/1078-0432.CCR-09-1095 Imaging, Diagnosis, Prognosis Circulating Tumor Cell as a Diagnostic Marker in Primary Lung Cancer Fumihiro Tanaka,1 Kazue Yoneda,1 Nobuyuki Kondo,1 Masaki Hashimoto,1 Teruhisa Takuwa,1 Seiji Matsumoto,1 Yoshitomo Okumura,1 Shakibur Rahman,1 Noriaki Tsubota,2 Tohru Tsujimura,3 Kozo Kuribayashi,4 Kazuya Fukuoka,4 Takashi Nakano,4 and Seiki Hasegawa1 Abstract Purpose: To investigate the diagnostic performance of circulating tumor cells (CTC) in discrimination between primary lung cancer and nonmalignant diseases as well as in prediction of distant metastasis. Patients and Methods: We prospectively evaluated CTCs in 7.5-mL samples of periph- eral blood sampled from patients with a suspicion or a diagnosis of primary lung can- cer. A semiautomated system was used to capture CTCs with an antibody against epithelial cell adhesion molecule. Results: Of 150 eligible patients, 25 were finally diagnosed as having nonmalignant dis- ease, and 125 were diagnosed as having primary lung cancer with (n = 31) or without (n = 94) distant metastasis. CTCs were detected in 30.6% of lung cancer patients and in 12.0% of nonmalignant patients. CTC count was significantly higher in lung cancer pa- tients than in nonmalignant patients, but a receiver operating characteristic (ROC) curve analysis showed an insufficient capability of the CTC test in discrimination be- tween lung cancer and nonmalignant diseases with an area under ROC curve of 0.598 (95% confidence interval, 0.488-0.708; P = 0.122). -

797 Circulating Tumor DNA and Circulating Tumor Cells for Cancer
Medical Policy Circulating Tumor DNA and Circulating Tumor Cells for Cancer Management (Liquid Biopsy) Table of Contents • Policy: Commercial • Coding Information • Information Pertaining to All Policies • Policy: Medicare • Description • References • Authorization Information • Policy History • Endnotes Policy Number: 797 BCBSA Reference Number: 2.04.141 Related Policies Biomarkers for the Diagnosis and Cancer Risk Assessment of Prostate Cancer, #336 Policy1 Commercial Members: Managed Care (HMO and POS), PPO, and Indemnity Plasma-based comprehensive somatic genomic profiling testing (CGP) using Guardant360® for patients with Stage IIIB/IV non-small cell lung cancer (NSCLC) is considered MEDICALLY NECESSARY when the following criteria have been met: Diagnosis: • When tissue-based CGP is infeasible (i.e., quantity not sufficient for tissue-based CGP or invasive biopsy is medically contraindicated), AND • When prior results for ALL of the following tests are not available: o EGFR single nucleotide variants (SNVs) and insertions and deletions (indels) o ALK and ROS1 rearrangements o PDL1 expression. Progression: • Patients progressing on or after chemotherapy or immunotherapy who have never been tested for EGFR SNVs and indels, and ALK and ROS1 rearrangements, and for whom tissue-based CGP is infeasible (i.e., quantity not sufficient for tissue-based CGP), OR • For patients progressing on EGFR tyrosine kinase inhibitors (TKIs). If no genetic alteration is detected by Guardant360®, or if circulating tumor DNA (ctDNA) is insufficient/not detected, tissue-based genotyping should be considered. Other plasma-based CGP tests are considered INVESTIGATIONAL. CGP and the use of circulating tumor DNA is considered INVESTIGATIONAL for all other indications. 1 The use of circulating tumor cells is considered INVESTIGATIONAL for all indications. -

Circulating Tumor Cells
The ability to isolate and molecularly analyze circulating tumor cells is becoming a reality. The Closest Exit May Be Behind You. Photograph courtesy of Craig Damlo. www.soapboxrocket.com. Circulating Tumor Cells: A Window Into Tumor Development and Therapeutic Effectiveness Gisela Cáceres, PhD, John A. Puskas, PhD, and Anthony M. Magliocco, MD Background: Circulating tumor cells (CTCs) are an important diagnostic tool for understanding the meta- static process and the development of cancer. Methods: This review covers the background, relevance, and potential limitations of CTCs as a measurement of cancer progression and how information derived from CTCs may affect treatment efficacy. It also highlights the difficulties of characterizing these rare cells due to the limited cell surface molecules unique to CTCs and each particular type of cancer. Results: The analysis of cancer in real time, through the measure of the number of CTCs in a “liquid” biopsy specimen, gives us the ability to monitor the therapeutic efficacy of treatments and possibly the metastatic potential of a tumor. Conclusions: Through novel and innovative techniques yielding encouraging results, including microfluidic techniques, isolating and molecularly analyzing CTCs are becoming a reality. CTCs hold promise for under- standing how tumors work and potentially aiding in their demise. Introduction States is attributed to cancer.2 The search for biomark- Cancer is a comprehensive term that includes a group ers that allow early detection and may therefore affect of diseases characterized by host cells growing with- the outcome of the disease is critical. The ability of out control. It has a multifactorial origin and, even cancer cells to spread in the body, producing metas- though many cancers are directly associated with risk tasis, is one of the most relevant characteristics of the factors in modern life, cancer is an ancient disease, disease and the cause of most cancer deaths. -
Liquid Biopsy Testing
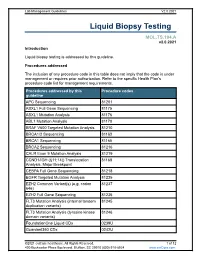
Lab Management Guidelines V2.0.2021 Liquid Biopsy Testing MOL.TS.194.A v2.0.2021 Introduction Liquid biopsy testing is addressed by this guideline. Procedures addressed The inclusion of any procedure code in this table does not imply that the code is under management or requires prior authorization. Refer to the specific Health Plan's procedure code list for management requirements. Procedures addressed by this Procedure codes guideline APC Sequencing 81201 ASXL1 Full Gene Sequencing 81175 ASXL1 Mutation Analysis 81176 ABL1 Mutation Analysis 81170 BRAF V600 Targeted Mutation Analysis 81210 BRCA1/2 Sequencing 81163 BRCA1 Sequencing 81165 BRCA2 Sequencing 81216 CALR Exon 9 Mutation Analysis 81219 CCND1/IGH (t(11;14)) Translocation 81168 Analysis, Major Breakpoint CEBPA Full Gene Sequencing 81218 EGFR Targeted Mutation Analysis 81235 EZH2 Common Variant(s) (e.g. codon 81237 646) EZH2 Full Gene Sequencing 81236 FLT3 Mutation Analysis (internal tandem 81245 duplication variants) FLT3 Mutation Analysis (tyrosine kinase 81246 domain variants) FoundationOne Liquid CDx 0239U Guardant360 CDx 0242U ©2021 eviCore healthcare. All Rights Reserved. 1 of 12 400 Buckwalter Place Boulevard, Bluffton, SC 29910 (800) 918-8924 www.eviCore.com Lab Management Guidelines V2.0.2021 Procedures addressed by this Procedure codes guideline Hematolymphoid Neoplasm Molecular 81450 Profiling; 5-50 genes IDH1 Mutation Analysis 81120 IDH2 Mutation Analysis 81121 IGH@/BCL2 (t(14;18)) Translocation 81278 Analysis, Major Breakpoint Region (MBR) and Minor Cluster Region (mcr) Breakpoints JAK2 Targeted Mutation Analysis (e.g 81279 exons 12 and 13) JAK2 V617F Targeted Mutation Analysis 81270 KIT Targeted Sequence Analysis 81272 KIT D816 Targeted Mutation Analysis 81273 KRAS Exon 2 Targeted Mutation Analysis 81275 KRAS Targeted Mutation Analysis, 81276 Additional Variants MGMT Promoter Methylation Analysis 81287 MLH1 Sequencing 81292 Molecular Tumor Marker Test 81400 81401 81402 g 81403 n i 81405 t 81406 s e T 81407 81408 y s 81479 p o Molecular Tumor Marker Test 88271 i B MPL Common Variants (e.g. -

Discrete Microfluidics for the Isolation of Circulating Tumor Cell
www.nature.com/npjprecisiononcology ARTICLE OPEN Discrete microfluidics for the isolation of circulating tumor cell subpopulations targeting fibroblast activation protein alpha and epithelial cell adhesion molecule Małgorzata A. Witek1,2,3, Rachel D. Aufforth4, Hong Wang3, Joyce W. Kamande3, Joshua M. Jackson1,2, Swathi R. Pullagurla1,2, Mateusz L. Hupert3,5, Jerry Usary6,7, Weiya Z. Wysham7,8, Dawud Hilliard7,9, Stephanie Montgomery9,10, Victoria Bae-Jump7,8, Lisa A. Carey7,11, Paola A. Gehrig7,8, Matthew I. Milowsky7, Charles M. Perou7, John T. Soper7,8, Young E. Whang7, Jen Jen Yeh4,7,12, George Martin13 and Steven A. Soper14,15,16 Circulating tumor cells consist of phenotypically distinct subpopulations that originate from the tumor microenvironment. We report a circulating tumor cell dual selection assay that uses discrete microfluidics to select circulating tumor cell subpopulations from a single blood sample; circulating tumor cells expressing the established marker epithelial cell adhesion molecule and a new marker, fibroblast activation protein alpha, were evaluated. Both circulating tumor cell subpopulations were detected in metastatic ovarian, colorectal, prostate, breast, and pancreatic cancer patients and 90% of the isolated circulating tumor cells did not co- express both antigens. Clinical sensitivities of 100% showed substantial improvement compared to epithelial cell adhesion molecule selection alone. Owing to high purity (>80%) of the selected circulating tumor cells, molecular analysis of both circulating tumor cell subpopulations was carried out in bulk, including next generation sequencing, mutation analysis, and gene expression. Results suggested fibroblast activation protein alpha and epithelial cell adhesion molecule circulating tumor cells are distinct subpopulations and the use of these in concert can provide information needed to navigate through cancer disease management challenges. -

Evaluation of Sensitivity and Specificity of Canpatroltm Technology For
Evaluation of sensitivity and specicity of CanPatrolTM technology for detection of circulating tumor cells in patients with non-small cell lung cancer Jingyao Li Army Medical University Yi Liao Army Medical University Yaling Ran Army Medical University Guiyu Wang Army Medical University Wei Wu Army Medical University Yang Qiu Army Medical University Jie Liu Army Medical University Ningyu Wen Army Medical University Tao Jing Army Medical University Haidong Wang ( [email protected] ) Third Military Medical University Southwest Hospital https://orcid.org/0000-0003-4409-4339 Shixin Zhang Army Medical University Research article Keywords: NSCLC, CTCs, CanPatrolTM, Sensitivity, Specicity Posted Date: May 10th, 2020 DOI: https://doi.org/10.21203/rs.3.rs-26700/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Version of Record: A version of this preprint was published on October 20th, 2020. See the published version at https://doi.org/10.1186/s12890-020-01314-4. Page 1/12 Abstract Background The early diagnosis of non-small cell lung cancer is of great signicance to the prognosis of patients. However, traditional histopathology and imaging screening have certain limitations. Therefore, new methods are urgently needed to make up for the current diagnostic defects. Objectives To evaluate of sensitivity and specicity of CanPatrol™ technology for detection of circulating tumor cells in patients with non-small cell lung cancer (NSCLC). Methods Non-interventional clinical research approach was used in this study. CTCs in the peripheral blood of 98 patients with NSCLC (including 48 patients in stage I, 13 in stage II, 29 in stage III and 8 in stage IV) and 38 patients with benign pulmonary diseases were collected by the latest typing of CanPatrol™ detection technology and nanomembrane ltration technology. -

A Review on Microdevices for Isolating Circulating Tumor Cells
micromachines Review A Review on Microdevices for Isolating Circulating Tumor Cells Kin Fong Lei 1,2 1 Graduate Institute of Biomedical Engineering, Chang Gung University, Taoyuan 333, Taiwan; kfl[email protected]; Tel.: +886-3-2118800 (ext. 5345) 2 Department of Radiation Oncology, Chang Gung Memorial Hospital, Linkou 333, Taiwan Received: 17 April 2020; Accepted: 20 May 2020; Published: 22 May 2020 Abstract: Cancer metastasis is the primary cause of high mortality of cancer patients. Enumeration of circulating tumor cells (CTCs) in the bloodstream is a very important indicator to estimate the therapeutic outcome in various metastatic cancers. The aim of this article is to review recent developments on the CTC isolation technologies in microdevices. Based on the categories of biochemical and biophysical isolation approaches, a literature review and in-depth discussion will be included to provide an overview of this challenging topic. The current excellent developments suggest promising CTC isolation methods in order to establish a precise indicator of the therapeutic outcome of cancer patients. Keywords: cancer metastasis; circulating tumor cells; CTC isolation; microdevices 1. Introduction According to clinical reports, cancer metastasis is the primary cause of high mortality of cancer patients [1,2]. Cancer metastasis is a multistep process of tumor cells escaping from the primary tumor site, entering into the bloodstream, arresting at the secondary site, extravasating into the tissue, and forming secondary tumor colonies [3,4]. During this process, the tumor cells that circulate in the bloodstream are called circulating tumor cells (CTCs). Because tissue biopsies are difficult to obtain from patients for evaluating the response of cancer therapy, CTCs are recognized as liquid biopsies to collect tumor cells from blood. -

Technical Validation of a New Microfluidic Device for Enrichment Of
www.nature.com/scientificreports OPEN Technical validation of a new microfuidic device for enrichment of CTCs from large volumes of blood by using bufy coats to mimic diagnostic leukapheresis products R. Guglielmi1, Z. Lai2, K. Raba3, G. van Dalum1, J. Wu1, B. Behrens1, A. A. S. Bhagat4,5, W. T. Knoefel1, R. P. L. Neves1,6 & N. H. Stoecklein1,6* Diagnostic leukapheresis (DLA) enables to sample larger blood volumes and increases the detection of circulating tumor cells (CTC) signifcantly. Nevertheless, the high excess of white blood cells (WBC) of DLA products remains a major challenge for further downstream CTC enrichment and detection. To address this problem, we tested the performance of two label-free CTC technologies for processing DLA products. For the testing purposes, we established fcollized bufy coats (BC) with a WBC composition similar to patient-derived DLA products. The mimicking-DLA samples (with up to 400 × 106 WBCs) were spiked with three diferent tumor cell lines and processed with two versions of a spiral microfuidic chip for label-free CTC enrichment: the commercially available ClearCell FR1 biochip and a customized DLA biochip based on a similar enrichment principle, but designed for higher throughput of cells. While the samples processed with FR1 chip displayed with increasing cell load signifcantly higher WBC backgrounds and decreasing cell recovery, the recovery rates of the customized DLA chip were stable, even if challenged with up to 400 × 106 WBCs (corresponding to around 120 mL peripheral blood or 10% of a DLA product). These results indicate that the further up-scalable DLA biochip has potential to process complete DLA products from 2.5 L of peripheral blood in an afordable way to enable high-volume CTC-based liquid biopsies. -

Circulating Tumour Cells from Patients with Colorectal Cancer Have
Gut Online First, published on July 25, 2016 as 10.1136/gutjnl-2016-311447 GI cancer ORIGINAL ARTICLE Circulating tumour cells from patients with Gut: first published as 10.1136/gutjnl-2016-311447 on 25 July 2016. Downloaded from colorectal cancer have cancer stem cell hallmarks in ex vivo culture Fanny Grillet,1,2,3 Elsa Bayet,1,2,3 Olivia Villeronce,1,2,3 Luke Zappia,4 Ebba Louise Lagerqvist,1,2,3 Sebastian Lunke,4 Emmanuelle Charafe-Jauffret,5 Kym Pham,4,6 Christina Molck,4 Nathalie Rolland,7 Jean François Bourgaux,8 Michel Prudhomme,9 Claire Philippe,9 Sophie Bravo,10 Jean Christophe Boyer,10 Lucile Canterel-Thouennon,11 Graham Roy Taylor,4 Arthur Hsu,4 Jean Marc Pascussi,1,2,3 Frédéric Hollande,1,2,3,4 Julie Pannequin1,2,3 ▸ Additional material is ABSTRACT published online only. To view Objective Although counting of circulating tumour Significance of this study please visit the journal online (http://dx.doi.org/10.1136/ cells (CTC) has attracted a broad interest as potential gutjnl-2016-311447). markers of tumour progression and treatment response, the lack of functional characterisation of these cells had What is already known on this subject? become a bottleneck in taking these observations to the ▸ fi Circulating tumour cells (CTCs) contain key For numbered af liations see clinic. Our objective was to culture these cells in order to end of article. prognostic markers for patients with metastatic understand them and exploit their therapeutic potential colorectal cancer (CRC). Correspondence to to the full. ▸ CTCs are scarce among blood cells and they are Dr Julie Pannequin, Design Here, hypothesising that some CTC potentially also heterogeneous. -

Circulating Cancer Stem Cell Markers in Breast Carcinomas: a Systematic Review Protocol Maryam Mansoori1, Zahra Madjd2*, Leila Janani3* and Arezoo Rasti4
Mansoori et al. Systematic Reviews (2017) 6:262 DOI 10.1186/s13643-017-0660-y PROTOCOL Open Access Circulating cancer stem cell markers in breast carcinomas: a systematic review protocol Maryam Mansoori1, Zahra Madjd2*, Leila Janani3* and Arezoo Rasti4 Abstract Background: Breast cancer is one of the most common types of cancer in women worldwide. Recent studies have provided strong support for the cancer stem cell (CSC) hypothesis, which suggests that many cancers, including breast cancer, are driven by a subpopulation of cells that display stem cell-like properties. The hypothesis that a subpopulation of circulating tumor cells (CTCs) possesses many CSC-like hallmarks is reinforced by the expression of related molecular markers between these two cell populations. The aim of this study is to systematically review primary studies and identify circulating CSC markers in breast cancer patients. Methods and design: Relevant observational studies evaluating the expression of circulating breast cancer stem cell markers through October 31, 2016, will be searched in PubMed, SCOPUS, Embase, ISI Web of Science, and Google Scholar with no restriction on language. Full copies of articles identified by the search and considered to meet the inclusion criteria will be obtained for data extraction and synthesis. Two quality assessment tools will be used for evaluating observational studies like case control, which are the Hoy et al. suggested tool and Newcastle-Ottawa Scale (NOS), respectively. Publication bias will be assessed by funnel plots or Egger’s test (i.e., plots of study results against precision), and data synthesis will be performed using Stata software (Stata Corp V.12, TX, USA).This systematic review will be reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). -

Circulating Tumor Cell Count
Circulating Tumor Cell Count Circulating tumor cell (CTC) count identies and quanties the EpCAM protein produced by epithelial tumor cells. Testing should be done prior to the initiation of therapy and serially during treatment. CTC count may also aid in determining prognosis and in Tests to Consider assessing treatment ecacy. Circulating Tumor Cell Count 0093399 Method: Immunomagnetic Separation/Immunouorescent Stain/Computer Disease Overview Assisted Analysis May be useful in predicting prognosis and Prognostic/Treatment Issues progression-free survival in patients with MBC, MCRC, and MPC and serve as an aid to evaluate and monitor response to therapy. CTC count in whole blood is an independent predictor of progression-free survival and overall survival; cutoffs vary by tumor cell type Metastatic breast cancer (MBC): >5 CTCs/7.5 mL of blood Metastatic colorectal cancer (MCRC): >3 CTCs/7.5 mL of blood Metastatic prostate cancer (MPC): >5 CTCs/7.5 mL of blood CTC count may indicate treatment ecacy Baseline CTC count should be obtained before initiation of therapy Serial CTC count testing may serve as a guide to therapy decisions and to assess prognosis Median Overall Survival Based on CTC Count MBC (mos) MCRC (mos) MPC (mos) CTC count < cutoff for all time points 22.6 18.6 >26 CTC count ≥ cutoff for all time points 4.1 3.9 6.8 CTC count ≥ cutoff at baseline and CTC < cutoff at nal draw 19.8 11.7 21.3 CTC count < cutoff at early draw and CTC count ≥ cutoff at nal draw 10.6 7.1 9.3 Miller, 2010 1 Physiology EpCAM is expressed by MBC, MCRC, -

Tumor Cells Circulate in the Peripheral Blood of All Major Carcinomas but Not in Healthy Subjects Or Patients with Nonmalignant Diseases
Vol. 10, 6897–6904, October 15, 2004 Clinical Cancer Research 6897 Tumor Cells Circulate in the Peripheral Blood of All Major Carcinomas but not in Healthy Subjects or Patients With Nonmalignant Diseases W. Jeffrey Allard,1 Jeri Matera,1 Conclusions: The CellSearch system can be standard- M. Craig Miller,1 Madeline Repollet,1 ized across multiple laboratories and may be used to deter- mine the clinical utility of CTCs. CTCs are extremely rare in Mark C. Connelly,1 Chandra Rao,1 1 2 healthy subjects and patients with nonmalignant diseases Arjan G. J. Tibbe, Jonathan W. Uhr, and but present in various metastatic carcinomas with a wide 1 Leon W. M. M. Terstappen range of frequencies. 1Immunicon Corporation, Huntingdon Valley, Pennsylvania; and 2Cancer Immunobiology Center and Departments of Microbiology, University of Texas Southwestern Medical Center at Dallas, INTRODUCTION Dallas, Texas There are accumulating reports of the isolation and char- acterization of circulating tumor cells [CTCs (1–18)]. The find- ings that CTCs can be found in patients before the primary ABSTRACT tumor is detected, CTCs are found in a significant proportion of Purpose: The purpose of this study was to determine the patients when a carcinoma recurs, and CTCs persist in some accuracy, precision, and linearity of the CellSearch system patients after removal of the primary tumor have been the and evaluate the number of circulating tumor cells (CTCs) impetus for continued studies of these tumor cells. Evidence that per 7.5 mL of blood in healthy subjects, patients with non- CTCs are derived from clones in the primary tumor (16) sug- malignant diseases, and patients with a variety of metastatic gests that they may reflect the tumor burden at all stages of carcinomas.