Here's the a to Z of Every Major Brand in Our Portfolio*
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Réglementation De La Pharmacie
R E C U E I L D E T E X T E S S U R L A P H A R M A C I E Mis à jour le 13 février 2017 par l’Inspection de la pharmacie P R É A M B U L E La réglementation relative à la pharmacie en vigueur en Nouvelle-Calédonie résulte de la coexistence des dispositions adoptées par la Nouvelle-Calédonie au titre de ses compétences en matières d’hygiène publique, de santé et de professions de la pharmacie1, et de celles adoptées par l’Etat au titre de ses compétences en matières de garanties des libertés publiques, de droit civil et de droit commercial2. Sur le contenu du recueil En 1954, la Nouvelle-Calédonie s’est vue étendre les articles L. 511 à L. 520 et L. 549 à L. 665 de l’ancien Livre V relatif à la Pharmacie du code de la santé publique métropolitain par la loi n° 54-418 du 15 avril 1954 étendant aux territoires d'outre-mer, au Togo et au Cameroun certaines dispositions du Code de la santé publique relatives à l'exercice de la pharmacie3, dont les modalités d’application ont été fixées par le décret modifié n° 55-1122 du 16 août 1955 fixant les modalités d'application de la loi n° 54-418 du 15 avril 1954 étendant aux territoires d'outre-mer, au Togo et au Cameroun certaines dispositions du code de la santé publique relatives à l'exercice de la pharmacie4. Depuis sont intervenues la loi- cadre Defferre5, la loi référendaire de 19886 et la loi organique n° 99-209 du 19 mars 1999 dont les apports ont eu pour résultat le transfert de ces articles de la compétence de l’Etat à la compétence de la Nouvelle-Calédonie, permettant à celle-ci de s’en approprier et de les modifier à sa guise par des délibérations du congrès de la Nouvelle-Calédonie7. -
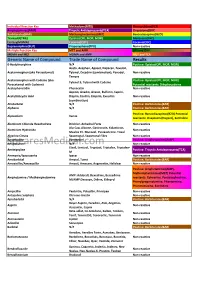
Cross Reaction Guide
Individual Reaction Key Methadone(MTD) Phencyclidine(PCP) Amphetamines(AMP) Tricyclic Antidepressants(TCA) Oxycodone(OXY) Barbiturates(BAR) Methamphetamines(MET) Benzodiazepines(BZO) Tramadol(TML) Opiates(OPI, MOP, MOR) Marijuana(THC) Ecstasy(MDMA) Cotinine(COT) Cocaine(COC) Buprenorphine(BUP) Propoxyphene(PPX) Non-reactive Multiple Reaction Key MET and AMP OPI and OXY MDMA and MET MDMA and AMP MET and TCA Generic Name of Compound Trade Name of Compound Results 6-Acetylmorphine N/A Positive: Opiates(OPI, MOP, MOR) Aceta, Acephen, Apacet, Dapacen, Feverall, Acetaminophen (aka Paracetamol) Tylenol, Excedrin (combination), Panadol, Non-reactive Tempra Acetaminophen with Codeine (aka Positive: Opiates(OPI, MOP, MOR) Tylenol 3, Tylenol with Codeine Paracetamol with Codeine) Potential reactants: Dihydrocodeine Acetophenetidin Phenacetin Non-reactive Aspirin, Anadin, Anasin, Bufferin, Caprin, Acetylsalicyclic Acid Disprin, Ecotrin, Empirin, Excedrin Non-reactive (combination) Allobarbital N/A Positive: Barbiturates(BAR) Alphenol N/A Positive: Barbiturates(BAR) Positive: Benzodiazepines(BZO) Potential Alprazolam Xanax reactants: Oxaprozin (Daypro), Sertraline Aluminum Chloride Hexahydrate Drichlor, Anhydrol Forte Non-reactive Alu-Cap, Alisone, Gastrocote, Kolanticon, Aluminum Hydroxide Non-reactive Maalox TC, Mucogel, Pyrogastrone, Topal Alverine Citrate Spasmonal, Spasmonal Fibre Non-reactive Amantadine Symmetrel Positive: Amphetamines(AMP) SpearesMedical.comAminopyrine N/A Non-reactive Elavil, Lentizol, Tryptizol, Triptafen, Triptafen- Amitriptyline -

In This Section
Strategic report In this section Chairman’s statement 2 CEO’s review 4 Business overview 6 The global context 8 Our business model 12 Our strategic priorities 14 How we performed 16 Risk management 18 Grow 20 Deliver 32 Simplify 44 Our financial architecture 48 Responsible business 50 Financial review 58 Strategic report Chairman’s statement Chairman’s statement To shareholders The value of the significant changes that have been made in recent years is evidenced in our performance this year “ Since Sir Andrew became It is clear from the following pages that Through the Audit & Risk Committee, we the Group made good progress against oversee the issues and challenges faced by CEO, the company has its strategy in 2013. management, and encourage the creation of an environment in which GSK can achieve The Board believes the business is seeing returned £30 billion its strategic ambitions in a responsible and the benefits of the significant changes the sustainable manner. to shareholders.” management team has driven over recent years to deliver sustainable growth, reduce risk and I have no doubt that commercial success is enhance returns to shareholders. directly linked to operating in a responsible way and which meets the changing expectations of The notably strong performance from the society. In this respect, the company continues R&D organisation in 2013 – with six major to adopt industry-leading positions on a range new product approvals in areas including of issues. respiratory disease, HIV and cancer – is critical to the longer-term prospects of the The announcement of plans during 2013 to Group. -

With Nicotine Replacement Therapies in Smoking Cessation Vol
Health Technology Assessment Health Technology Health Technology Assessment 2008; Vol. 12: No. 2 2008; 12: No. 2 Vol. ‘Cut down to quit’ with nicotine replacement therapies in smoking cessation ‘Cut down to quit’ with nicotine replacement therapies in smoking cessation: a systematic review of effectiveness and economic analysis D Wang, M Connock, P Barton, A Fry-Smith, P Aveyard and D Moore Feedback The HTA Programme and the authors would like to know your views about this report. The Correspondence Page on the HTA website (http://www.hta.ac.uk) is a convenient way to publish your comments. If you prefer, you can send your comments to the address below, telling us whether you would like us to transfer them to the website. We look forward to hearing from you. February 2008 The National Coordinating Centre for Health Technology Assessment, Mailpoint 728, Boldrewood, Health Technology Assessment University of Southampton, NHS R&D HTA Programme Southampton, SO16 7PX, UK. HTA Fax: +44 (0) 23 8059 5639 Email: [email protected] www.hta.ac.uk http://www.hta.ac.uk ISSN 1366-5278 HTA How to obtain copies of this and other HTA Programme reports. An electronic version of this publication, in Adobe Acrobat format, is available for downloading free of charge for personal use from the HTA website (http://www.hta.ac.uk). A fully searchable CD-ROM is also available (see below). Printed copies of HTA monographs cost £20 each (post and packing free in the UK) to both public and private sector purchasers from our Despatch Agents. Non-UK purchasers will have to pay a small fee for post and packing. -

PDF (Irish Pharmacist January 2020)
EXCLUSIVE COVERAGE FROM THE RECENT IRISH MEDICATION SAFETY NETWORK ANNUAL CONFERENCE ABOUT-TURN WE LOOK AT WHAT MIGHT BE NEXT FOR PHARMACISTS FOLLOWING THE REVERSAL IN PROPOSED FEE CUTS DUMB AND DUMBER? ARE PHARMACISTS A LITTLE LACKING IN GREY MATTER WHEN IT COMES TO PRICING, ASKS FINTAN MOORE PLUS CLINICAL CONTENT: ASTHMA ❋ COPD ❋ EYE CARE ❋ SMOKING CESSATION TENDER CARE AT Every Change For topical use only. Cleanse and dry the affected area before applying. A copy of the summary of product characteristics is available upon request. The active ingredient in Caldesene Medicated Powder is Calcium Undecylenate 10% w/w, 20g, 55g, 100g pack size. For supply through general sale. PA 126/152/1 PA Holder: Clonmel Healthcare Ltd., Waterford Road, Clonmel, Co. Tipperary. Date Prepared: October 2019. 2019/ADV/CAL/150H NEW TREATS HEARTBURN AND ACID REFLUX. ONE TABLET PER DAY. LASTS 24 HOURS. AVAILABLE IN PACKS OF 7s AND 14s. Marketed by CCF:22656 Date of preparation: (10-19) ABBREVIATED PRESCRIBING INFORMATION Product Name: Emazole Control 20 mg Gastro-Resistant Tablets Composition: Each tablet contains 20 mg esomeprazole (as magnesium dihydrate). Description: Light pink oval film coated tablet. Indication(s): Proton Pump Inhibitor (PPI): Short-term treatment of reflux symptoms (e.g. heartburn and acid regurgitation) in adults. Dosage: Swallow tablets whole with liquid, do not chew or crush. Disperse in half a glass of non-carbonated water if difficulty in swallowing. Stir until tablets disintegrate, drink liquid with pellets immediately or within 15 min, or administer through a gastric tube. Do not chew or crush pellets. Adults: The recommended dose is 20 mg esomeprazole (one tablet) per day. -

Glaxosmithkline Plc Annual Report for the Year Ended 31St December 2000
GlaxoSmithKline 01 GlaxoSmithKline plc Annual Report for the year ended 31st December 2000 Contents Report of the Directors 02 Financial summary 03 Joint statement by the Chairman and the Chief Executive Officer 05 Description of business 29 Corporate governance 37 Remuneration report 47 Operating and financial review and prospects 69 Financial statements 70 Directors’ statements of responsibility 71 Report by the auditors 72 Consolidated statement of profit and loss 72 Consolidated statement of total recognised gains and losses 74 Consolidated statement of cash flow 76 Consolidated balance sheet 76 Reconciliation of movements in equity shareholders’ funds 77 Company balance sheet 78 Notes to the financial statements 136 Group companies 142 Principal financial statements in US$ 144 Financial record 153 Investor information 154 Shareholder return 156 Taxation information for shareholders 157 Shareholder information 158 Share capital 160 Cross reference to Form 20-F 162 Glossary of terms The Annual Report was approved by the Board 163 Index of Directors on 22nd March 2001 and published on 12th April 2001. Contact details 02 GlaxoSmithKline Financial summary 2000 1999 Increase Business performance £m £m CER % £ % Sales 18,079 16,164 9 12 Trading profit 5,026 4,378 12 15 Profit before taxation 5,327 4,708 11 13 Earnings/Net income 3,697 3,222 13 15 Earnings per Ordinary Share 61.0p 52.7p 14 16 Total results Profit before taxation 6,029 4,236 Earnings/Net income 4,154 2,859 Earnings per Ordinary Share 68.5p 46.7p Business performance: results exclude merger items and restructuring costs; 1999 sales and trading profit exclude the Healthcare Services businesses which were disposed of in 1999. -

Paul M Mahan Matthews
17 April 2015 PAUL McMAHAN MATTHEWS [email protected] PRIMARY APPOINTMENTS July, 2014- Edmond and Lily Safra Professor of Translational Neuroscience and Therapeutics, Division of Brain Sciences, Department of Medicine, Imperial College London Secured endowment for, and first holder of, new Chair associated with the Divisional Head and posts (Edmond and Lily Safra Scholars) accompanying its foundation. April, 2012- Professor and Head, Division of Brain Sciences, Department of Medicine, Imperial College London Leading a new integration of over 70 academics all clinical and basic neurosciences, neuropsychopharmacology, neurology and mental health within a Division of 200 staff in the Faculty of Medicine with senior management responsibilities across the three academic hospital sites of Imperial College, London. Additional responsibilities as Deputy Director (2008- ) of the Wellcome Trust- GSK Translational Medicine Training Programme, Deputy Director (2011- ) of the BRC Institute for Translational Medicine and Therapeutics, Biomedical Research Centre (BRC) Theme Lead for Neuroscience, co-Director of the Neurotechnology Network and the EPSRC Centre for Doctoral Training in Neurology and Research Advisory Board for the Data Science Institute. July, 2012- February 2014 Vice-President for Integrative Medicines Development, Neurosciences and Medicine Development Lead, GlaxoSmithKline (joint appointment with Imperial College) Leading late phase development programme. Senior advisor to the Head of Neurosciences and China R and D with specific -

Formulation of Novel Buccal Mucosal Drug Delivery Systems for Nicotine Replacement Therapy (Nrt)
FORMULATION OF NOVEL BUCCAL MUCOSAL DRUG DELIVERY SYSTEMS FOR NICOTINE REPLACEMENT THERAPY (NRT). OBINNA CHIKWADO OKEKE A thesis submitted in partial fulfilment of the requirements of the University of Greenwich for the Degree of Doctor of Philosophy January 2017 DECLARATION “I certify that this work has not been accepted in substance for any degree, and is not concurrently being submitted for any purpose. I also declare that this work is the result of my own investigations except where otherwise identified by references and that I have not plagiarised another’s work”. Mr. Obinna C. Okeke (Candidate) Dr J.S. Boateng (First supervisor) i ACKNOWLEDGEMENTS I would like to express my sincere gratitude to my supervisor, Dr Joshua Boateng, for his enormous support, encouragement and general guidance throughout my research. His constant support and encouragement has led me to believe in myself and give out my best. I also appreciate the University of Greenwich for providing the facilities and student support within this period. I would like to acknowledge Dr Ian Slipper for all SEM and XRPD techniques as well as Devyani Amin for HPLC analysis. Thanks to all University staff for supporting my program. I also want to extend my deepest gratitude to my family. Their support and encouragement have led me to the person I am today. I am grateful to Dr Sam and Samantha Owusu-Ware and family for their support. Finally, to all my roommates, officemates, library IT and assistance team, project students under my supervision and fellow postgraduate students, I want to say I big thank you for your support. -

First Quarter 2019
Press release First quarter 2019 Issued: Wednesday, 1 May 2019, London U.K. GSK delivers sales of £7.7 billion +6% AER, +5% CER Total EPS 16.8p, +50% AER, +42% CER; Adjusted EPS 30.1p, +22% AER, +18% CER Financial highlights • Pharmaceuticals sales £4.2 billion, +4% AER, +2% CER; Vaccines £1.5 billion, +23% AER, +20% CER; Consumer Healthcare £2.0 billion, flat AER, +1% CER. • Total Group operating margin 18.6%. Adjusted Group operating margin 28.2%, +1.6 percentage points AER, +1.0 percentage point CER (Pharmaceuticals 29.8%; Vaccines 40.3%; Consumer Healthcare 21.7%). Benefits from strong sales growth and phasing of R&D. • Total EPS 16.8p, +50% AER, +42% CER. • Adjusted EPS 30.1p, +22% AER, +18% CER, driven by strong operating performance, continued financial efficiencies, reduction in minority share and a one-off benefit to associates. • Net cash flow from operations £663 million. Free cash flow £165 million. • 19p dividend declared for the quarter; continue to expect 80p for full year 2019. • 2019 Guidance reaffirmed. Product and pipeline highlights • Total HIV sales £1.1 billion, +7% AER, +4% CER, including Juluca sales of £70 million. - Dovato (dolutegravir+lamivudine), first once-daily 2-drug regimen for treatment-naive HIV patients, launched in US. - Long-acting cabotegravir+rilpivirine filed in the US for treatment of HIV. • Total new Respiratory product sales £631 million, +29% AER, +25% CER, including Trelegy £87 million; Nucala £152 million. • Shingrix sales £357 million driven by continued strong launch execution in US. • Continued -

Today's Research Tomorrow's Cure
2018 Heart Research Institute Annual Review Accelerating Research Tomorrow’s cure Tomorrow’s Today’s research Today’s 2018 Heart Research Institute Annual Review Hello Future Accelerating 2018 Heart Research Institute Annual Review Research ACCELERATING Discoveries into therapies Knowledge into prevention Breakthroughs into cures Students into leaders Collaborations into partnerships 4 2018 Heart Research Institute Annual Review Inspiring Leaders 2018 Heart Research Institute Annual Review CONTENTS 06 HRI in 2018 08 Chairman’s Report 2018 10 Director of Cardiovascular Research Report 12 2018 Research and Media Highlights 16 Applied Materials Group 18 Atherosclerosis and Vascular Remodelling Group 20 Cardiac Imaging Group 22 Cardiometabolic Disease Group 24 Cardiovascular Medical Devices Group 26 Cell Therapeutics Group 28 Clinical Research Group 30 Haematology Research Group 32 Heart Rhythm and Stroke Prevention Group 34 High Blood Pressure Group 36 Thrombosis Group 38 Vascular Complications Group 40 Vascular Immunology Group 42 Inflammation and Fibrosis Research 44 PhD Students 45 Select Prizes and Awards 46 Select Conferences and Presentations 50 Select Publications 56 Board of Governors 59 International Board of Governors 60 Members of the Institute 62 Fundraising Report 66 Operations Report 6 2018 Heart Research Institute Annual Review The mission of the Heart Research Institute (HRI) is to prevent death and suffering from cardiovascular disease through an understanding of the biological processes that cause atherosclerosis and thrombosis, the major underlying causes of most heart attacks and strokes. 20 18 SHORT TERM LONG TERM The major short-term focus of our research There are four long-term objectives is to understand the development and for our research: progression of atherothrombotic conditions • To investigate mechanisms in which the arteries are narrowed and contributing to the pathogenesis restricted due to a build-up of fatty of cardiovascular disease. -

Building Innovation, Performance and Trust
Building Innovation, Performance and Trust Emma Walmsley, CEO Cautionary statement regarding forward-looking statements This presentation may contain forward-looking statements. Forward-looking statements give the Group’s current expectations or forecasts of future events. An investor can identify these statements by the fact that they do not relate strictly to historical or current facts. They use words such as ‘anticipate’, ‘estimate’, ‘expect’, ‘intend’, ‘will’, ‘project’, ‘plan’, ‘believe’, ‘target’ and other words and terms of similar meaning in connection with any discussion of future operating or financial performance. In particular, these include statements relating to future actions, prospective products or product approvals, future performance or results of current and anticipated products, sales efforts, expenses, the outcome of contingencies such as legal proceedings, and financial results. Other than in accordance with its legal or regulatory obligations (including under the Market Abuse Regulations, UK Listing Rules and the Disclosure and Transparency Rules of the Financial Conduct Authority), the Group undertakes no obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise. Investors should, however, consult any additional disclosures that the Group may make in any documents which it publishes and/or files with the US Securities and Exchange Commission (SEC). All investors, wherever located, should take note of these disclosures. Accordingly, no assurance can be given that any particular expectation will be met and investors are cautioned not to place undue reliance on the forward-looking statements. Forward-looking statements are subject to assumptions, inherent risks and uncertainties, many of which relate to factors that are beyond the Group’s control or precise estimate. -

2009 Nhamcs Micro-Data File Documentation Page 1
2009 NHAMCS MICRO-DATA FILE DOCUMENTATION PAGE 1 ABSTRACT This material provides documentation for users of the public use micro-data files of the 2009 National Hospital Ambulatory Medical Care Survey (NHAMCS). NHAMCS is a national probability sample survey of visits to hospital outpatient and emergency departments, conducted by the National Center for Health Statistics, Centers for Disease Control and Prevention. The survey is a component of the National Health Care Surveys, which measure health care utilization across a variety of health care providers. There are two micro-data files produced from NHAMCS, one for outpatient department records and one for emergency department records. Section I of this documentation, “Description of the National Hospital Ambulatory Medical Care Survey,” includes information on the scope of the survey, the sample, field activities, data collection procedures, medical coding procedures, and population estimates. Section II provides detailed descriptions of the contents of each file’s data record by location. Section III contains marginal data for selected items on each file. The appendixes contain sampling errors, instructions and definitions for completing the Patient Record forms, and lists of codes used in the survey. PAGE 2 2009 NHAMCS MICRO-DATA FILE DOCUMENTATION SUMMARY OF CHANGES FOR 2009 The 2009 NHAMCS Emergency Department and Outpatient Department public use micro-data files are, for the most part, similar to the 2008 files, but there are some important changes. These are described in more detail below and reflect changes to the survey instrument, the Patient Record form. Emergency Departments 1. New or Modified Items a. In item 1, Patient Information, there is a new checkbox item “Arrival by Ambulance.” This replaces the 2008 item, “Mode of Arrival.” b.