(Lepidoptera: Noctuidae), on Resident Communities of Pest and Natural Enemies in Maize Fields in Kenya
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Abundance of Sesamia Nonagrioides (Lef.) (Lepidoptera: Noctuidae) on the Edges of the Mediterranean Basin
Hindawi Publishing Corporation Psyche Volume 2012, Article ID 854045, 7 pages doi:10.1155/2012/854045 Review Article Abundance of Sesamia nonagrioides (Lef.) (Lepidoptera: Noctuidae) on the Edges of the Mediterranean Basin Matilde Eizaguirre1 and Argyro A. Fantinou2 1 Department of Crop and Forest Sciences, University of Lleida, 25198 Lleida, Spain 2 Laboratory of Ecology, Agricultural University of Athens, 11855 Athens, Greece Correspondence should be addressed to Matilde Eizaguirre, [email protected] Received 5 September 2011; Revised 17 November 2011; Accepted 21 November 2011 Academic Editor: Matilda Savopoulou-Soultani Copyright © 2012 M. Eizaguirre and A. A. Fantinou. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Organisms inhabiting seasonal environments are able to synchronize their life cycles with seasonal cycles of biotic and abiotic factors. Diapause, a state of low metabolic activity and developmental arrest, is used by many insect species to cope with adverse conditions. Sesamia nonagrioides is a serious pest of corn in the Mediterranean regions and Central Africa. It is multivoltine, with two to four generations per year, that overwinters as mature larva in the northern of the Sahara desert. Our purpose was to compare theresponseoftheS. nonagrioides populations occurring in the broader circum-Mediterranean area, with particular attention to the diapause period and the different numbers of generations per season. To this end, we tried to determine whether populations in the area differ in their response to photoperiod and whether we can foresee the number of generations in different areas. -

Archiv Für Naturgeschichte
© Biodiversity Heritage Library, http://www.biodiversitylibrary.org/; www.zobodat.at Bericht über die wissenschaftlichen Leistungen im Gebiete der Entomologie während der Jahre 1859 und 1860. (Zweite Hälfte). Von Dr. A. Gerstaecker in Berlin. Hymenoptera. Auf die Verschiedenheiten, welche die an der Costa der Hymenopteren-Hinterflüg-el befindlichen Häkchen, durch welche bekanntlich der Schluss der Flügel während des Fluges der Aderflügler bedingt wird , sowohl in Zahl als Anordnung darbieten, hat Miss Staveley in einer durch Abbildungen illustrirten Abhandlung „Observations on the neuration of the bind wings of Hymenopterous Insects, and on the hooks which join the forc and bind wings together in flight" (Transact. Linnean soc. of London XXIII. 1. p. 125— 137. tab.l6u. 17) hingewiesen. Diese Abhandlung ist eine weitere Ausführung einer schon von J. E. Gray (Annais of nat. bist. V. p. 339 ff.) mitgethcilton und von derselben Verfasserin herrührenden hürzeren Notiz : „On the hooks on the front edge of the hinder wings of certain Hymenoptera," in welcher zunächst nur auf die Modifika- tionen jener Flügelhäkchen bei einigen Ichneurnoniden hingewiesen wird. — in der genannten grösseren Abhand- lung geht die Verf. zunächst auf das bisher wenig beachtete Geäder der Hinterflügcl ein und glaubt die Verschieden- heiten desselben , besonders in Bezug auf das Verhalten der Costa , drei Categorieen zuertheilen zu müssen (die © Biodiversity Heritage Library, http://www.biodiversitylibrary.org/; www.zobodat.at Gerstaecker: Bericht über die Wissenschaft). -

Downloaded from the Same Database
G C A T T A C G G C A T genes Article Genetic Basis of Maize Resistance to Multiple Insect Pests: Integrated Genome-Wide Comparative Mapping and Candidate Gene Prioritization A. Badji 1,* , D. B. Kwemoi 2, L. Machida 3 , D. Okii 1, N. Mwila 1, S. Agbahoungba 4, F. Kumi 5 , A. Ibanda 1 , A. Bararyenya 1, M. Solemanegy 1, T. Odong 1, P. Wasswa 1, M. Otim 2, G. Asea 2, M. Ochwo-Ssemakula 1, H. Talwana 1, S. Kyamanywa 1 and P. Rubaihayo 1 1 Department of Agricultural Production, Makerere Univesity, P.O. Box 7062 Kampala, Uganda; [email protected] (D.O.); [email protected] (N.M.); [email protected] (A.I.); [email protected] (A.B.); [email protected] (M.S.); [email protected] (T.O.); [email protected] (P.W.); [email protected] (M.O.-S.); [email protected] (H.T.); [email protected] (S.K.); [email protected] (P.R.) 2 Cereals Program, National Crop Resource Research Institute, P.O. Box 7084 Kampala, Uganda; [email protected] (D.B.K.); [email protected] (M.O.); [email protected] (G.A.) 3 Alliance Bioversity International-CIAT, P.O. Box 24384 Kampala, Uganda; [email protected] 4 Laboratory of Applied Ecology, University of Abomey-Calavi, 01BP 526 Cotonou, Benin; [email protected] 5 Department of Crop Science, University of Cape Coast, P.O. Box 5007 PMB Cape Coast, Ghana; [email protected] * Correspondence: [email protected] Received: 19 May 2020; Accepted: 1 June 2020; Published: 24 June 2020 Abstract: Several species of herbivores feed on maize in field and storage setups, making the development of multiple insect resistance a critical breeding target. -

The Role of Wild Host Plants in the Abundance of Lepidopteran Stem Borers Along Altitudinal Gradients in Kenya
Ann. soc. enromol. Fr. (n.s.), 2006, 42 (3-4) : 363-370 ARTICLE The role ofwild host plants in the abundance oflepidopteran stem borers along altitudinal gradient in Kenya GEORGE O. ONG'AMO(I), BRUNO P. LE RD(I), STI~,PHANE DUPAS(l), PASCAL MOYAL(l), ERIC MUCHUGU(3), PAUL-ANDRE CALATAYUD(I) & JEAN-FRAN<;:OIS SILVAIN(2) (I) Nocruid Stem Borer Biodiversity Project (NSBB), Insrirur de Recherche pour le Developpernenr I International Cenrre of Insect Physiology and Ecology (IRD/ICIPE), P.O. Box 30772, Nairobi, Kenya (2) IRD, UR R072 clo CNRS, UPR 9034, Laboraroire Evolution, Genomes et Speciarion, avenue de la Terrasse, 91198 Gif/Yvene, France (31 Stem borer Biological Control Project (ICIPE), P.O. Box 30772, Nairobi, Kenya Abstract. Presence of wild host plants of stem borers in cereal-growing areas has been considered as reservoirs of lepidopteran stem borers, responsible for attack of crops during the growing season. Surveys to catalogue hosts and borers as well as to assess the abundance of the hosts were carried out during the cropping and non-cropping seasons in different agro-ecological zones along varying altitude gradient in Kenya. A total of 61 stem borer species belonging to families Noctuidae (25), Crambidae (14), Pyralidae (9), Tortricidae (11) and Cossidae (2) were recovered from 42 wild plant species. Two noctuids, Busseola fusca (Fuller), Sesamia calamistis Hampson, and two crambids, Chilo partellus (Swinhoe) and Chilo orichalcociliellus (Strand) were the four main borer species found associated with maize plants. In the wild, B. fusca was recovered from a limited number of host plant species and among them were Sorghum arundinaceum (Desvaux) Stapf, Setaria megaphylla (Steudel) 1. -

INSECT RESISTANCE MONITORING REPORT for Sesamia Nonagrioides ASSOCIATED with MON 810 MAIZE CULTIVATION in the EU
INSECT RESISTANCE MONITORING REPORT FOR Sesamia nonagrioides ASSOCIATED WITH MON 810 MAIZE CULTIVATION IN THE EU Season 2017 CONTENTS 1. Introduction 2 2. Materials and methods 2.1. Insect collection 4 2.2. Insect culture 5 2.3. Quality of the laboratory strain 5 2.4. Cry1Ab protein 6 2.5. Bioassays 7 2.5.1. Susceptibility of the reference strains of S. nonagrioides 7 and O. nubilalis to the Cry1Ab protein in dose-response bioassays 2.5.2. Susceptibility of S. nonagrioides to the Cry1Ab protein in 8 diagnostic concentration bioassays 2.5.3. Larval development on MON 810 tissue 8 2.6. Statistical analysis 9 3. Results and Discussion 3.1. Collection of larvae 9 3.2. Susceptibility of the reference strains to the C ry1Ab protein in 10 dose-response bioassays 3.3. Diagnostic concentration bioassays 11 3.4. Confirmatory experiment: Development of larvae on MON 810 12 leaves 4. Summary of results 13 5. Concluding remarks 14 6. References 15 7. Tables and Figures 17-26 Annex I Stepwise approach followed to do the bioassays 27 Annex II Map with the sampling locations of S. nonagrioides larvae in 2017 28-29 Annex III Map with the sampling locations of O. nubilalis larvae in 2017 30-31 1 1. Introduction Maize containing event MON 810 is transgenic improved maize expressing the Cry1Ab protein derived from Bacillus thuringiensis subsp. kurstaki , and conferring protection against certain lepidopteran insect pests such as Ostrinia nubilalis and Sesamia nonagrioides . Resistance development in targeted lepidopteran pests is a potential concern arising from the widespread cultivation of MON 810 maize varieties. -

Response of Chilo Partellus (Lepidoptera: Crambidae) to Bt Maize in South Africa
Response of Chilo partellus (Lepidoptera: Crambidae) to Bt maize in South Africa J Vorster orcid.org/0000-0001-8126-6860 Dissertation submitted in fulfilment of the requirements for the Masters degree in Environmental Science at the North-West University Supervisor: Prof J van den Berg Co-supervisor: Prof MJ du Plessis Assistant supervisor: Dr A Erasmus Graduation May 2018 23441674 ACKNOWLEDGEMENTS This dissertation would not have been possible without the help of so many people. I am blessed and very grateful to have them in my life. I would like to start with our God Almighty and our Saviour who bestowed upon me the strength, wisdom and peace of mind to finish this project and who also have sent me these blessed people in my life. I would like to thank Prof. Johnnie van den Berg and Dr. Annemie Erasmus for all the guidance and support they have given me. You taught me that small things can make a big difference. Statistics can be difficult sometimes and I thank Prof. Hannalene du Plessis and Prof. Suria Elis for the help with the statistics. Thank you to all the staff at the ARC-GCI that assisted me with the trials in the lab and the planting. Elrine Strydom, Mabel du Toit, Heidi Meyer and Ursula du Plessis, thank you for the countless after hours we had to spend and for the warm hearted kindness you have given me. I would also like to thank my parents whom I dearly love for all the encouragement and motivation to do my best. You taught me that hard work does not come easily, but the fruit that you pick from it is what motivates us. -

First Detection of a Sesamia Nonagrioides Resistance Allele to Bt Maize in Europe Received: 1 November 2017 Ana M
www.nature.com/scientificreports OPEN First detection of a Sesamia nonagrioides resistance allele to Bt maize in Europe Received: 1 November 2017 Ana M. Camargo1,2, David A. Andow2, Pedro Castañera1 & Gema P. Farinós 1 Accepted: 9 February 2018 The Ebro Valley (Spain) is the only hotspot area in Europe where resistance evolution of target pests Published: xx xx xxxx to Cry1Ab protein is most likely, owing to the high and regular adoption of Bt maize (>60%). The high-dose/refuge (HDR) strategy was implemented to delay resistance evolution, and to be efective it requires the frequency of resistance alleles to be very low (<0.001). An F2 screen was performed in 2016 to estimate the frequency of resistance alleles in Sesamia nonagrioides from this area and to evaluate if the HDR strategy is still working efectively. Out of the 137 isofemale lines screened on Cry1Ab maize leaf tissue, molted larvae and extensive feeding were observed for two consecutive generations in one line, indicating this line carried a resistance allele. The frequency of resistance alleles in 2016 was 0.0036 (CI 95% 0.0004–0.0100), higher but not statistically diferent from the value obtained in 2004– 2005. Resistance does not seem to be evolving faster than predicted by a S. nonagrioides resistance evolution model, but the frequency of resistance is now triple the value recommended for an efective implementation of the HDR strategy. Owing to this, complementary measures should be considered to further delay resistance evolution in the Ebro Valley. Te commercial use of genetically engineered (GE) crops in Europe has been controversial. -

Assessing the Long-Term Welfare Effects of the Biological Control Of
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by ICRISAT Open Access Repository Agriculture, Ecosystems and Environment 230 (2016) 10–23 Contents lists available at ScienceDirect Agriculture, Ecosystems and Environment journa l homepage: www.elsevier.com/locate/agee Assessing the long-term welfare effects of the biological control of cereal stemborer pests in East and Southern Africa: Evidence from Kenya, Mozambique and Zambia a,b, a,c b Soul-kifouly G. Midingoyi *, Hippolyte D. Affognon , Ibrahim Macharia , a,d a,d a e a,f Georges Ong’amo , Esther Abonyo , Gerphas Ogola , Hugo De Groote , Bruno LeRu a International Centre of Insect Physiology and Ecology (icipe), P.O. Box 30772-00100, Nairobi, Kenya b School of Agriculture and Enterprise Development, Kenyatta University, P.O. Box 43844-00100, Nairobi, Kenya c International Crops Research Institute for the Semi-Arid Tropics (ICRISAT), BP 320, Bamako, Mali d School of Biological Sciences, University of Nairobi, P.O. Box 30197, Nairobi, Kenya e International Maize and Wheat Improvement Centre (CIMMYT), P.O. Box 1041-00621, Nairobi, Kenya f UMR Laboratoire Evolution, Génomes, Comportement et Ecologie, groupe IRD, Diversité, Ecologie et Evolution des Insectes Tropicaux, UPR 9034, 22 CNRS, 91198 Gif-sur-Yvette, France A R T I C L E I N F O A B S T R A C T Article history: Received 20 January 2016 The International Centre of Insect Physiology and Ecology (icipe), undertook a biological control (BC) Received in revised form 18 May 2016 programme for control of stemborers from 1993 to 2008, to reduce cereal yield losses due to stemborer Accepted 22 May 2016 attack in East and Southern Africa. -

Downloaded from BOLD Or Requested from Other Authors
www.nature.com/scientificreports OPEN Towards a global DNA barcode reference library for quarantine identifcations of lepidopteran Received: 28 November 2018 Accepted: 5 April 2019 stemborers, with an emphasis on Published: xx xx xxxx sugarcane pests Timothy R. C. Lee 1, Stacey J. Anderson2, Lucy T. T. Tran-Nguyen3, Nader Sallam4, Bruno P. Le Ru5,6, Desmond Conlong7,8, Kevin Powell 9, Andrew Ward10 & Andrew Mitchell1 Lepidopteran stemborers are among the most damaging agricultural pests worldwide, able to reduce crop yields by up to 40%. Sugarcane is the world’s most prolifc crop, and several stemborer species from the families Noctuidae, Tortricidae, Crambidae and Pyralidae attack sugarcane. Australia is currently free of the most damaging stemborers, but biosecurity eforts are hampered by the difculty in morphologically distinguishing stemborer species. Here we assess the utility of DNA barcoding in identifying stemborer pest species. We review the current state of the COI barcode sequence library for sugarcane stemborers, assembling a dataset of 1297 sequences from 64 species. Sequences were from specimens collected and identifed in this study, downloaded from BOLD or requested from other authors. We performed species delimitation analyses to assess species diversity and the efectiveness of barcoding in this group. Seven species exhibited <0.03 K2P interspecifc diversity, indicating that diagnostic barcoding will work well in most of the studied taxa. We identifed 24 instances of identifcation errors in the online database, which has hampered unambiguous stemborer identifcation using barcodes. Instances of very high within-species diversity indicate that nuclear markers (e.g. 18S, 28S) and additional morphological data (genitalia dissection of all lineages) are needed to confrm species boundaries. -

The Apameini of Israel (Lepidoptera: Noctuidae) SHILAP Revista De Lepidopterología, Vol
SHILAP Revista de Lepidopterología ISSN: 0300-5267 [email protected] Sociedad Hispano-Luso-Americana de Lepidopterología España Kravchenko, V. D.; Fibiger, M.; Mooser, J.; Junnila, A.; Müller, G. C. The Apameini of Israel (Lepidoptera: Noctuidae) SHILAP Revista de Lepidopterología, vol. 36, núm. 142, junio, 2008, pp. 253-259 Sociedad Hispano-Luso-Americana de Lepidopterología Madrid, España Available in: http://www.redalyc.org/articulo.oa?id=45512540015 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative SHILAP Nº 142 9/6/08 11:52 Página 253 SHILAP Revta. lepid., 36 (142), junio 2008: 253-259 CODEN: SRLPEF ISSN:0300-5267 The Apameini of Israel (Lepidoptera: Noctuidae) V. D. Kravchenko, M. Fibiger, J. Mooser, A. Junnila & G. C. Müller Abstract In Israel, 20 species of tribe Apameini belonging to 10 genera have been found to date. Four species are endemic of the Levant (Sesamia ilonae, Luperina kravchenkoi, Gortyna gyulaii and Lenisa wiltshirei). Others are mostly Palaearctic, Mediterranean, Iranian and Irano-Turanian elements. Grassland species of the Apameini are mainly associated with the Temperate region and are univoltine with highest rate of occurrence in May, or in autumn. Most wetland and oases species are multivoltine and occur in oases and riverbeds over the country, though few of the species are extremely localized. The S. ilonae is presently known only from northern area of the Sea of Galilee, while A. deserticola is from oases of the Dead Sea area. -
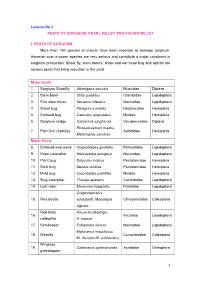
Lecture No 3 PESTS of SORGHUM, PEARL MILLET and FINGER MILLET
Lecture No 3 PESTS OF SORGHUM, PEARL MILLET AND FINGER MILLET I. PESTS OF SORGHUM More than 150 species of insects have been reported to damage sorghum. However over a dozen species are very serious and constitute a major constraint in sorghum production. Shoot fly, stem borers, shoot and ear head bug and aphids are serious pests that bring reduction in the yield. Major pests 1. Sorghum Shootfly Atherigona soccata Muscidae Diptera 2. Stem borer Chilo partellus Crambidae Lepidoptera 3. Pink stem borer Sesamia inferens Noctuidae Lepidoptera 4 Shoot bug Peregrinus maidis Delphacidae Hemiptera 5. Earhead bug Calocoris angustatus Miridae Hemiptera 6. Sorghum midge Contarinia sorghicola Cecidomyiidae Diptera Rhopalosiphum maidis, 7. Plant lice (Aphids) Aphididae Hemiptera Melanaphis sacchari Minor Pests 8. Earhead web worm Cryptoblabes gnidiella Pyraustidae Lepidoptera 9. Gram caterpillar Helicoverpa armigera Noctuidae Lepidoptera 10. Plant bug Dolycoris indicus Pentatomidae Hemiptera 11. Stink bug Nezara viridula Pentatomidae Hemiptera 12. Mirid bug Creontiades pallidifer Miridae Hemiptera 13. Slug caterpillar Thosea apierens Cochlididae Lepidoptera 14. Leaf roller Marasmia trapezalis Pyralidae Lepidoptera Cryptocephalus 15. Flea beetle schestedii, Monolepta Chrysomelidae Coleoptera signata Red hairy Amsacta albistriga, 16. Arctiidae Lepidoptera caterpillar A. moorei 17. Semilooper Eublemma silicula Noctuidae Lepidoptera Myllocerus maculosus 18. Weevils Curculionidae Coleoptera M. discolor,M. subfaciatus Wingless 19. Colemania sphenaroides Acrididae Orthoptera grasshopper MAJOR PESTS 1.Sorghum Shootfly: Atherigona soccata (Muscidae: Diptera) Distribution and status Maharashtra, Andhra Pradesh, Tamil Nadu and Karnataka Host range: Maize, ragi, bajra, rice, wheat and grasses Damage symptoms The maggot on hatching migrates to the upper surface of leaf and enters between the leaf sheath and stem. -

Crambidae Biosecurity Occurrence Background Subfamilies Short Description Diagnosis
Diaphania nitidalis Chilo infuscatellus Crambidae Webworms, Grass Moths, Shoot Borers Biosecurity BIOSECURITY ALERT This Family is of Biosecurity Concern Occurrence This family occurs in Australia. Background The Crambidae is a large, diverse and ubiquitous family of moths that currently comprises 11,500 species globally, with at least half that number again undescribed. The Crambidae and the Pyralidae constitute the superfamily Pyraloidea. Crambid larvae are concealed feeders with a great diversity in feeding habits, shelter building and hosts, such as: leaf rollers, shoot borers, grass borers, leaf webbers, moss feeders, root feeders that shelter in soil tunnels, and solely aquatic life habits. Many species are economically important pests in crops and stored food products. Subfamilies Until recently, the Crambidae was treated as a subfamily under the Pyralidae (snout moths or grass moths). Now they form the superfamily Pyraloidea with the Pyralidae. The Crambidae currently consists of the following 14 subfamilies: Acentropinae Crambinae Cybalomiinae Glaphyriinae Heliothelinae Lathrotelinae Linostinae Midilinae Musotiminae Odontiinae Pyraustinae Schoenobiinae Scopariinae Spilomelinae Short Description Crambid caterpillars are generally cylindrical, with a semiprognathous head and only primary setae (Fig 1). They are often plainly coloured (Fig. 16, Fig. 19), but can be patterned with longitudinal stripes and pinacula that may give them a spotted appearance (Fig. 10, Fig. 11, Fig. 14, Fig. 22). Prolegs may be reduced in borers (Fig. 16). More detailed descriptions are provided below. This factsheet presents, firstly, diagnostic features for the Pyraloidea (Pyralidae and Crambidae) and then the Crambidae. Information and diagnostic features are then provided for crambids listed as priority biosecurity threats for northern Australia.