Article Processing Charges for This Open-Access Depth, Rendering Deeper and Older OM Bioavailable for Mi- Publication Were Covered by a Research Crobial Decomposition
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Atlantic Constrain Laurentide Ice Sheet Extent During Marine Isotope Stage 3
Sea-level records from the U.S. mid- Atlantic constrain Laurentide Ice Sheet extent during Marine Isotope Stage 3 The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Pico, T, J. R. Creveling, and J. X. Mitrovica. 2017. “Sea-level records from the U.S. mid-Atlantic constrain Laurentide Ice Sheet extent during Marine Isotope Stage 3.” Nature Communications 8 (1): 15612. doi:10.1038/ncomms15612. http://dx.doi.org/10.1038/ ncomms15612. Published Version doi:10.1038/ncomms15612 Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:33490941 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA ARTICLE Received 7 Dec 2016 | Accepted 11 Apr 2017 | Published 30 May 2017 DOI: 10.1038/ncomms15612 OPEN Sea-level records from the U.S. mid-Atlantic constrain Laurentide Ice Sheet extent during Marine Isotope Stage 3 T. Pico1, J.R. Creveling2 & J.X. Mitrovica1 The U.S. mid-Atlantic sea-level record is sensitive to the history of the Laurentide Ice Sheet as the coastline lies along the ice sheet’s peripheral bulge. However, paleo sea-level markers on the present-day shoreline of Virginia and North Carolina dated to Marine Isotope Stage (MIS) 3, from 50 to 35 ka, are surprisingly high for this glacial interval, and remain unexplained by previous models of ice age adjustment or other local (for example, tectonic) effects. -

The Easternmost Occurrence of Mammut Pacificus (Proboscidea: Mammutidae), Based on a Partial Skull from Eastern Montana, USA
The easternmost occurrence of Mammut pacificus (Proboscidea: Mammutidae), based on a partial skull from eastern Montana, USA Andrew T. McDonald1, Amy L. Atwater2, Alton C. Dooley Jr1 and Charlotte J.H. Hohman2,3 1 Western Science Center, Hemet, CA, United States of America 2 Museum of the Rockies, Montana State University, Bozeman, MT, United States of America 3 Department of Earth Sciences, Montana State University, Bozeman, MT, United States of America ABSTRACT Mammut pacificus is a recently described species of mastodon from the Pleistocene of California and Idaho. We report the easternmost occurrence of this taxon based upon the palate with right and left M3 of an adult male from the Irvingtonian of eastern Montana. The undamaged right M3 exhibits the extreme narrowness that characterizes M. pacificus rather than M. americanum. The Montana specimen dates to an interglacial interval between pre-Illinoian and Illinoian glaciation, perhaps indicating that M. pacificus was extirpated in the region due to habitat shifts associated with glacial encroachment. Subjects Biogeography, Evolutionary Studies, Paleontology, Zoology Keywords Mammut pacificus, Mammutidae, Montana, Irvingtonian, Pleistocene INTRODUCTION Submitted 7 May 2020 The recent recognition of the Pacific mastodon (Mammut pacificus (Dooley Jr et al., 2019)) Accepted 3 September 2020 as a new species distinct from and contemporaneous with the American mastodon Published 16 November 2020 (M. americanum) revealed an unrealized complexity in North American mammutid Corresponding author evolution during the Pleistocene. Dooley Jr et al. (2019) distinguished M. pacificus from Andrew T. McDonald, [email protected] M. americanum by a suite of dental and skeletal features: (1) upper third molars (M3) Academic editor and lower third molars (m3) much narrower relative to length in M. -

An Optimized Scheme of Lettered Marine Isotope Substages for the Last 1.0 Million Years, and the Climatostratigraphic Nature of Isotope Stages and Substages
1 Quaternary Science Reviews Achimer March 2015, Volume 111 Pages 94-106 http://dx.doi.org/10.1016/j.quascirev.2015.01.012 http://archimer.ifremer.fr http://archimer.ifremer.fr/doc/00251/36216/ © 2015 Elsevier Ltd. All rights reserved. An optimized scheme of lettered marine isotope substages for the last 1.0 million years, and the climatostratigraphic nature of isotope stages and substages Railsback L. Bruce 1, * , Gibbard Philip L. 2, Head Martin J. 3, Voarintsoa Ny Riavo G. 1, Toucanne Samuel 4 1 Department of Geology, University of Georgia, Athens, GA 30602-2501, USA 2 Department of Geography, University of Cambridge, Downing Street, Cambridge CB2 3EN, England, UK 3 Department of Earth Sciences, Brock University, 500 Glenridge Avenue, St. Catharines, Ontario L2S 3A1, Canada 4 IFREMER, Laboratoire Environnements Sédimentaires, BP70, 29280 Plouzané, France * Corresponding author : L. Bruce Railsback, Tel.: +1 706 542 3453; fax: +1 706 542 2652 ; email address : [email protected] Abstract : A complete and optimized scheme of lettered marine isotope substages spanning the last 1.0 million years is proposed. Lettered substages for Marine Isotope Stage (MIS) 5 were explicitly defined by Shackleton (1969), but analogous substages before or after MIS 5 have not been coherently defined. Short-term discrete events in the isotopic record were defined in the 1980s and given decimal-style numbers, rather than letters, but unlike substages they were neither intended nor suited to identify contiguous intervals of time. Substages for time outside MIS 5 have been lettered, or in some cases numbered, piecemeal and with conflicting designations. We therefore propose a system of lettered substages that is complete, without missing substages, and optimized to match previous published usage to the maximum extent possible. -

Review of the Early–Middle Pleistocene Boundary and Marine Isotope Stage 19 Martin J
Head Progress in Earth and Planetary Science (2021) 8:50 Progress in Earth and https://doi.org/10.1186/s40645-021-00439-2 Planetary Science REVIEW Open Access Review of the Early–Middle Pleistocene boundary and Marine Isotope Stage 19 Martin J. Head Abstract The Global Boundary Stratotype Section and Point (GSSP) defining the base of the Chibanian Stage and Middle Pleistocene Subseries at the Chiba section, Japan, was ratified on January 17, 2020. Although this completed a process initiated by the International Union for Quaternary Research in 1973, the term Middle Pleistocene had been in use since the 1860s. The Chiba GSSP occurs immediately below the top of Marine Isotope Substage (MIS) 19c and has an astronomical age of 774.1 ka. The Matuyama–Brunhes paleomagnetic reversal has a directional midpoint just 1.1 m above the GSSP and serves as the primary guide to the boundary. This reversal lies within the Early– Middle Pleistocene transition and has long been favoured to mark the base of the Middle Pleistocene. MIS 19 occurs within an interval of low-amplitude orbital eccentricity and was triggered by an obliquity cycle. It spans two insolation peaks resulting from precession minima and has a duration of ~ 28 to 33 kyr. MIS 19c begins ~ 791– 787.5 ka, includes full interglacial conditions which lasted for ~ 8–12.5 kyr, and ends with glacial inception at ~ 774– 777 ka. This inception has left an array of climatostratigraphic signals close to the Early–Middle Pleistocene boundary. MIS 19b–a contains a series of three or four interstadials often with rectangular-shaped waveforms and marked by abrupt (< 200 year) transitions. -

The Last Interglacial-Glacial Cycle (MIS 5-2) Re-Examined Based on Long Proxy Records from Central and Northern Europe
Technical Report TR-13-02 The Last Interglacial-Glacial cycle (MIS 5-2) re-examined based on long proxy records from central and northern Europe Karin F Helmens Department of Physical Geography and Quaternary Geology, Stockholm University October 2013 Svensk Kärnbränslehantering AB Swedish Nuclear Fuel and Waste Management Co Box 250, SE-101 24 Stockholm Phone +46 8 459 84 00 ISSN 1404-0344 Tänd ett lager: SKB TR-13-02 P, R eller TR. ID 1360839 The Last Interglacial-Glacial cycle (MIS 5-2) re-examined based on long proxy records from central and northern Europe Karin F Helmens Department of Physical Geography and Quaternary Geology, Stockholm University October 2013 This report concerns a study which was conducted for SKB. The conclusions and viewpoints presented in the report are those of the author. SKB may draw modified conclusions, based on additional literature sources and/or expert opinions. A pdf version of this document can be downloaded from www.skb.se. Abstract A comparison is made between five Late Pleistocene terrestrial proxy records from central, temper- ate and northern, boreal Europe. The records comprise the classic proxy records of La Grande Pile (E France) and Oerel (N Germany) and more recently obtained records from Horoszki Duże (E Poland), Sokli (N Finland) and Lake Yamozero (NW Russia). The Sokli sedimentary sequence from the central area of Fennoscandian glaciation has escaped major glacial erosion in part due to non-typical bedrock conditions. Multi-proxy studies on the long Sokli sequence drastically change classic ideas of glaciation, vegetation and climate in northern Europe during the Late Pleistocene. -
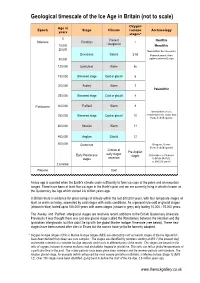
Ice Age Timescale for Website
Geological timescale of the Ice Age in Britain (not to scale) Oxygen Age in Epoch Stage Climate isotope Archaeology years stages* 0 Present Neolithic Holocene Flandrian 1 10,000 interglacial Mesolithic 20,000 Neanderthals become extinct Devensian Glacial 2-5d Modern humans (Homo 80,000 sapiens) arrive in Europe 120,000 Ipswichian Warm 5e 150,000 Unnamed stage Cold or glacial 6 200,000 ‘Aveley’ Warm 7 Palaeolithic 250,000 Unnamed stage Cold or glacial 8 Pleistocene 300,000 ‘Purfleet’ Warm 9 Neanderthals (Homo 350,000 Unnamed stage Cold or glacial 10 neanderthalensis) evolve from Homo heidelbergensis 400,000 Hoxnian Warm 11 450,000 Anglian Glacial 12 500,000 Cromerian Boxgrove, Sussex (Homo heidelbergensis) Climate of Pre-Anglian early stages Early Pleistocene stages First evidence of humans stages uncertain in Britain (Norfolk) (c. 800,000 years?) 2.6 million Pliocene Cool An ice age is a period when the Earth's climate cools sufficiently to form ice caps at the poles and on mountain ranges. There have been at least five ice ages in the Earth’s past and we are currently living in what is known as the Quaternary Ice Age which started 2.6 million years ago. In Britain there is evidence for great swings of climate within the last 500,000 years, with four temperate stages at least as warm as today, separated by cold stages with arctic conditions. As a general rule cold or glacial stages (shown in blue) lasted up to 100,000 years with warm stages (shown in grey) only lasting 10,000 - 15,000 years. -

The Sea-Level Highstand Correlated to Marine Isotope Stage (MIS) 7 in the Coastal Plain of the State of Rio Grande Do Sul, Brazil
Anais da Academia Brasileira de Ciências (2014) 86(4): 1573-1595 (Annals of the Brazilian Academy of Sciences) Printed version ISSN 0001-3765 / Online version ISSN 1678-2690 http://dx.doi.org/10.1590/0001-3765201420130274 www.scielo.br/aabc The sea-level highstand correlated to marine isotope stage (MIS) 7 in the coastal plain of the state of Rio Grande do Sul, Brazil RENATO P. LOPES1, SERGIO R. DILLENBURG1, CESAR L. SCHULTZ1, JORGE FERIGOLO1,2, ANA MARIA RIBEIRO1,2, JAMIL C. PEREIRA1,3, ELIZETE C. HOLANDA4, VANESSA G. PITANA1 and LEONARDO KERBER1 1Programa de Pós-Graduação em Geociências, Universidade Federal do Rio Grande do Sul/UFRGS, Av. Bento Gonçalves, 9500, Agronomia, 91540-000 Porto Alegre, RS, Brasil 2Fundação Zoobotânica do Rio Grande do Sul, Museu de Ciências Naturais, Seção de Paleontologia, Av. Salvador França, 1427, Jardim Botânico, 90690-000 Porto Alegre, RS, Brasil 3Museu Coronel Tancredo Fernandes de Mello, Rua Barão do Rio Branco, 467, 96230-000 Santa Vitória do Palmar, RS, Brasil 4Universidade Federal de Roraima, Instituto de Geociências, Departamento de Geologia, Av. Cap. Ene Garcez, 2413, Sala 11, Aeroporto, 69304-000 Boa Vista, RR, Brasil Manuscript received on August 8, 2013; accepted for publication on February 17, 2014 ABSTRACT The coastal plain of the state of Rio Grande do Sul, in southern Brazil, includes four barrier-lagoon depositional systems formed by successive Quaternary sea-level highstands that were correlated to marine isotope stages (MIS) 11, 9, 5 and 1, despite the scarcity of absolute ages. This study describes a sea-level highstand older than MIS 5, based on the stratigraphy, ages and fossils of the shallow marine facies found in coastal barrier (Barrier II). -

Palaeogeographical Changes in Response to Glacial–Interglacial Cycles, As Recorded in Middle and Late Pleistocene Seismic Stratigraphy, Southern North Sea
JOURNAL OF QUATERNARY SCIENCE (2020) 35(6) 760–775 ISSN 0267-8179. DOI: 10.1002/jqs.3230 Palaeogeographical changes in response to glacial–interglacial cycles, as recorded in Middle and Late Pleistocene seismic stratigraphy, southern North Sea STEPHEN J. EATON,1* DAVID M. HODGSON,1 NATASHA L. M. BARLOW,1 ESTELLE E. J. MORTIMER1 and CLAIRE L. MELLETT2 1School of Earth and Environment, University of Leeds, UK 2Wessex Archaeology, Salisbury, Wiltshire, UK Received 20 September 2019; Revised 16 June 2020; Accepted 17 June 2020 ABSTRACT: Offshore stratigraphic records from the North Sea contain information to reconstruct palaeo‐ice‐sheet extent and understand sedimentary processes and landscape response to Pleistocene glacial–interglacial cycles. We document three major Middle to Late Pleistocene stratigraphic packages over a 401‐km2 area (Norfolk Vanguard/ Boreas Offshore Wind Farm), offshore East Anglia, UK, through the integration of 2D seismic, borehole and cone penetration test data. The lowermost unit is predominantly fluviatile [Yarmouth Roads Formation, Marine Isotope Stage (MIS) 19–13], including three northward‐draining valleys. The middle unit (Swarte Bank Formation) records the southernmost extent of tunnel valley‐fills in this area of the North Sea, providing evidence for subglacial conditions most likely during the Anglian stage (MIS 12) glaciation. The Yarmouth Roads and Swarte Bank deposits are truncated and overlain by low‐energy estuarine silts and clays (Brown Bank Formation; MIS 5d–4). Smaller scale features, including dune‐scale bedforms, and abrupt changes in cone penetration test parameters, provide evidence for episodic changes in relative sea level within MIS 5. The landscape evolution recorded in deposits of ~MIS 19–5 are strongly related to glacial–interglacial cycles, although a distinctive aspect of this low‐relief ice‐marginal setting are opposing sediment transport directions under contrasting sedimentary process regimes. -

Co-Evolution of Millennial and Orbital Variability in Quaternary Climate
Clim. Past, 12, 1805–1828, 2016 www.clim-past.net/12/1805/2016/ doi:10.5194/cp-12-1805-2016 © Author(s) 2016. CC Attribution 3.0 License. Mode transitions in Northern Hemisphere glaciation: co-evolution of millennial and orbital variability in Quaternary climate David A. Hodell1 and James E. T. Channell2 1Godwin Laboratory for Palaeoclimate Research, Department of Earth Sciences, Downing Street, Cambridge, CB2 3EQ, UK 2Department of Geological Sciences, University of Florida, 241 Williamson Hall, PO Box 112120, Gainesville 32611, USA Correspondence to: David A. Hodell ([email protected]) Received: 4 March 2016 – Published in Clim. Past Discuss.: 24 March 2016 Revised: 11 August 2016 – Accepted: 19 August 2016 – Published: 7 September 2016 Abstract. We present a 3.2 Myr record of stable isotopes Sea Drilling Project, DSDP, and Ocean Drilling Program, and physical properties at IODP Site U1308 (reoccupation ODP) have provided a detailed history of Northern Hemi- of DSDP Site 609) located within the ice-rafted detritus sphere glaciation during the Pliocene and Quaternary. The (IRD) belt of the North Atlantic. We compare the isotope intensification of Northern Hemisphere glaciation began in and lithological proxies at Site U1308 with other North At- the latest Pliocene (∼ 2.7 Ma) and the intensity, shape, and lantic records (e.g., sites 982, 607/U1313, and U1304) to re- duration of glacial–interglacial cycles changed during the construct the history of orbital and millennial-scale climate Quaternary. The average climate state evolved towards gen- variability during the Quaternary. The Site U1308 record erally colder conditions with larger and thicker ice sheets, documents a progressive increase in the intensity of North- and the spectral character of climate variability shifted from ern Hemisphere glacial–interglacial cycles during the late a dominant period of 41 kyr to a quasi-period between 80 Pliocene and Quaternary, with mode transitions at ∼ 2.7, 1.5, and 120 kyr. -

Simulating Marine Isotope Stage 7 with a Coupled Climate-Ice Sheet
https://doi.org/10.5194/cp-2020-46 Preprint. Discussion started: 27 March 2020 c Author(s) 2020. CC BY 4.0 License. Simulating Marine Isotope Stage 7 with a coupled climate-ice sheet model Dipayan Choudhury1,2, Axel Timmermann1,2, Fabian Schloesser3, Malte Heinemann4 and David Pollard5 1Center for Climate Physics, Institute for Basic Science (IBS), Busan 46241, South Korea 2Pusan National University, Busan 46241, South Korea 5 3International Pacific Research Center, University of Hawaii at Manoa, Honolulu, HI 96822, USA 4Institute of Geosciences, Kiel University, 24118, Kiel, Germany 5Earth and Environmental Systems Institute, Pennsylvania State University, Pennsylvania 16802, USA Correspondence to: Dipayan Choudhury ([email protected]) 10 Abstract. It is widely accepted that orbital variations are responsible for the generation of glacial cycles during the late Pleistocene. However, the relative contributions of the orbital forcing compared to CO2 variations and other feedback mechanisms causing the waxing and waning of ice-sheets have not been fully understood. Testing theories of ice-ages beyond statistical inferences, requires numerical modeling experiments that capture key features of glacial transitions. Here, we focus on the glacial build-up from Marine Isotope Stage (MIS) 7 to 6 covering the period from 240-170 ka (thousand years before 15 present). This transition from interglacial to glacial conditions includes one of the fastest Pleistocene glaciation/deglaciation events which occurred during MIS 7e-7d-7c (236-218ka). Using a newly developed three-dimensional coupled atmosphere- ocean-vegetation-ice-sheet model (LOVECLIP), we simulate the transient evolution of northern and southern hemisphere ice- sheets during the MIS 7-6 period in response to orbital and greenhouse-gas forcing. -

Palaeoclimate
6 Palaeoclimate Coordinating Lead Authors: Eystein Jansen (Norway), Jonathan Overpeck (USA) Lead Authors: Keith R. Briffa (UK), Jean-Claude Duplessy (France), Fortunat Joos (Switzerland), Valérie Masson-Delmotte (France), Daniel Olago (Kenya), Bette Otto-Bliesner (USA), W. Richard Peltier (Canada), Stefan Rahmstorf (Germany), Rengaswamy Ramesh (India), Dominique Raynaud (France), David Rind (USA), Olga Solomina (Russian Federation), Ricardo Villalba (Argentina), De’er Zhang (China) Contributing Authors: J.-M. Barnola (France), E. Bauer (Germany), E. Brady (USA), M. Chandler (USA), J. Cole (USA), E. Cook (USA), E. Cortijo (France), T. Dokken (Norway), D. Fleitmann (Switzerland, Germany), M. Kageyama (France), M. Khodri (France), L. Labeyrie (France), A. Laine (France), A. Levermann (Germany), Ø. Lie (Norway), M.-F. Loutre (Belgium), K. Matsumoto (USA), E. Monnin (Switzerland), E. Mosley-Thompson (USA), D. Muhs (USA), R. Muscheler (USA), T. Osborn (UK), Ø. Paasche (Norway), F. Parrenin (France), G.-K. Plattner (Switzerland), H. Pollack (USA), R. Spahni (Switzerland), L.D. Stott (USA), L. Thompson (USA), C. Waelbroeck (France), G. Wiles (USA), J. Zachos (USA), G. Zhengteng (China) Review Editors: Jean Jouzel (France), John Mitchell (UK) This chapter should be cited as: Jansen, E., J. Overpeck, K.R. Briffa, J.-C. Duplessy, F. Joos, V. Masson-Delmotte, D. Olago, B. Otto-Bliesner, W.R. Peltier, S. Rahmstorf, R. Ramesh, D. Raynaud, D. Rind, O. Solomina, R. Villalba and D. Zhang, 2007: Palaeoclimate. In: Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change [Solomon, S., D. Qin, M. Manning, Z. Chen, M. Marquis, K.B. -

A Review of Early Weichselian Climate (MIS 5D-A) in Europe
Technical Report TR-13-03 A review of Early Weichselian climate (MIS 5d-a) in Europe Barbara Wohlfarth Department of Geological Sciences, Stockholm University June 2013 Svensk Kärnbränslehantering AB Swedish Nuclear Fuel and Waste Management Co Box 250, SE-101 24 Stockholm Phone +46 8 459 84 00 ISSN 1404-0344 Tänd ett lager: SKB TR-13-03 P, R eller TR. ID 1358661 A review of Early Weichselian climate (MIS 5d-a) in Europe Barbara Wohlfarth Department of Geological Sciences, Stockholm University June 2013 This report concerns a study which was conducted for SKB. The conclusions and viewpoints presented in the report are those of the author. SKB may draw modified conclusions, based on additional literature sources and/or expert opinions. A pdf version of this document can be downloaded from www.skb.se. Preface This document contains information on climate and environmental conditions during the complex transition from warm interglacial to cold glacial conditions at the beginning of the Weichselian period, as recorded in marine, terrestrial and glacial archives. The information is of relevance for the analysis of long-term repository safety in e.g. SKB’s safety assessment SR-PSU. In particular, the information will be used in the report “Climate and climate-related issues for the safety assessment SR-PSU” and in the “SR-PSU Main report”. Stockholm, June 2013 Jens-Ove Näslund Person in charge of the SKB climate programme SKB TR-13-03 3 Summary This report addresses the transition from the last interglacial into the last glacial period in Europe, which corresponds to the time interval between approximately 122,000 and 70,000 years before present.