Guidance on the Use of Antipsychotics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Management of Chronic Problems
MANAGEMENT OF CHRONIC PROBLEMS INTERACTIONS BETWEEN ALCOHOL AND DRUGS A. Leary,* T. MacDonald† SUMMARY concerned. Alcohol may alter the effects of the drug; drug In western society alcohol consumption is common as is may change the effects of alcohol; or both may occur. the use of therapeutic drugs. It is not surprising therefore The interaction between alcohol and drug may be that concomitant use of these should occur frequently. The pharmacokinetic, with altered absorption, metabolism or consequences of this combination vary with the dose of elimination of the drug, alcohol or both.2 Alcohol may drug, the amount of alcohol taken, the mode of affect drug pharmacokinetics by altering gastric emptying administration and the pharmacological effects of the drug or liver metabolism. Drugs may affect alcohol kinetics by concerned. Interactions may be pharmacokinetic or altering gastric emptying or inhibiting gastric alcohol pharmacodynamic, and while coincidental use of alcohol dehydrogenase (ADH).3 This may lead to altered tissue may affect the metabolism or action of a drug, a drug may concentrations of one or both agents, with resultant toxicity. equally affect the metabolism or action of alcohol. Alcohol- The results of concomitant use may also be principally drug interactions may differ with acute and chronic alcohol pharmacodynamic, with combined alcohol and drug effects ingestion, particularly where toxicity is due to a metabolite occurring at the receptor level without important changes rather than the parent drug. There is both inter- and intra- in plasma concentration of either. Some interactions have individual variation in the response to concomitant drug both kinetic and dynamic components and, where this is and alcohol use. -

)&F1y3x PHARMACEUTICAL APPENDIX to THE
)&f1y3X PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE )&f1y3X PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 3 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. Product CAS No. Product CAS No. ABAMECTIN 65195-55-3 ACTODIGIN 36983-69-4 ABANOQUIL 90402-40-7 ADAFENOXATE 82168-26-1 ABCIXIMAB 143653-53-6 ADAMEXINE 54785-02-3 ABECARNIL 111841-85-1 ADAPALENE 106685-40-9 ABITESARTAN 137882-98-5 ADAPROLOL 101479-70-3 ABLUKAST 96566-25-5 ADATANSERIN 127266-56-2 ABUNIDAZOLE 91017-58-2 ADEFOVIR 106941-25-7 ACADESINE 2627-69-2 ADELMIDROL 1675-66-7 ACAMPROSATE 77337-76-9 ADEMETIONINE 17176-17-9 ACAPRAZINE 55485-20-6 ADENOSINE PHOSPHATE 61-19-8 ACARBOSE 56180-94-0 ADIBENDAN 100510-33-6 ACEBROCHOL 514-50-1 ADICILLIN 525-94-0 ACEBURIC ACID 26976-72-7 ADIMOLOL 78459-19-5 ACEBUTOLOL 37517-30-9 ADINAZOLAM 37115-32-5 ACECAINIDE 32795-44-1 ADIPHENINE 64-95-9 ACECARBROMAL 77-66-7 ADIPIODONE 606-17-7 ACECLIDINE 827-61-2 ADITEREN 56066-19-4 ACECLOFENAC 89796-99-6 ADITOPRIM 56066-63-8 ACEDAPSONE 77-46-3 ADOSOPINE 88124-26-9 ACEDIASULFONE SODIUM 127-60-6 ADOZELESIN 110314-48-2 ACEDOBEN 556-08-1 ADRAFINIL 63547-13-7 ACEFLURANOL 80595-73-9 ADRENALONE -

THE HARD TRUTH ABOUT PROKINETIC MEDICATION USE in PETS Introduction Pathophysiology/Etiology to That Observed in Dogs
VETTALK Volume 15, Number 04 American College of Veterinary Pharmacists THE HARD TRUTH ABOUT PROKINETIC MEDICATION USE IN PETS Introduction Pathophysiology/Etiology to that observed in dogs. It can be The moving topic of this Vet Talk As with most diseases in the veteri- due to a trichobezoar, dehydration, newsletter will be prokinetic medica- nary world, the etiology and patho- obesity, old age, diabetes, immobility, tions. The availability of information physiology of constipation are varied pain from trauma to the low back, on the many prokinetic agents is var- depending on the species being dis- bladder infection, or an anal sac infec- ied at best so an overall consensus of cussed, where in their gastrointestinal tion. In cases that are more chronic, prokinetic medications will be as- tract the problem is occurring, and underlying disease such as colitis or sessed in this article, hopefully giving any accompanying comorbid condi- Irritable Bowel Syndrome (IBS) may better insight to practitioners about tions. be the culprit. On the other hand, the which agents to use in their patients. cause may be idiopathic which is Canines: In man’s best friend, consti- frustrating for both veterinarian and Prevalence pation has many origins. A dog’s patient since this form is most diffi- Chronic constipation and gastroin- digestive tract itself is complex but cult to treat. testinal stasis are highly debilitating ultimately the mass movements and conditions that not only affect human haustral contractions from the large Equines: Despite their large size, patients but our four legged patients intestine (colon), propel feces into the horses have incredibly delicate diges- as well! Though this condition is rectum stimulating the internal anal tive systems. -

Pharmacokinetics and Pharmacology of Drugs Used in Children
Drug and Fluid Th erapy SECTION II Pharmacokinetics and Pharmacology of Drugs Used CHAPTER 6 in Children Charles J. Coté, Jerrold Lerman, Robert M. Ward, Ralph A. Lugo, and Nishan Goudsouzian Drug Distribution Propofol Protein Binding Ketamine Body Composition Etomidate Metabolism and Excretion Muscle Relaxants Hepatic Blood Flow Succinylcholine Renal Excretion Intermediate-Acting Nondepolarizing Relaxants Pharmacokinetic Principles and Calculations Atracurium First-Order Kinetics Cisatracurium Half-Life Vecuronium First-Order Single-Compartment Kinetics Rocuronium First-Order Multiple-Compartment Kinetics Clinical Implications When Using Short- and Zero-Order Kinetics Intermediate-Acting Relaxants Apparent Volume of Distribution Long-Acting Nondepolarizing Relaxants Repetitive Dosing and Drug Accumulation Pancuronium Steady State Antagonism of Muscle Relaxants Loading Dose General Principles Central Nervous System Effects Suggamadex The Drug Approval Process, the Package Insert, and Relaxants in Special Situations Drug Labeling Opioids Inhalation Anesthetic Agents Morphine Physicochemical Properties Meperidine Pharmacokinetics of Inhaled Anesthetics Hydromorphone Pharmacodynamics of Inhaled Anesthetics Oxycodone Clinical Effects Methadone Nitrous Oxide Fentanyl Environmental Impact Alfentanil Oxygen Sufentanil Intravenous Anesthetic Agents Remifentanil Barbiturates Butorphanol and Nalbuphine 89 A Practice of Anesthesia for Infants and Children Codeine Antiemetics Tramadol Metoclopramide Nonsteroidal Anti-infl ammatory Agents 5-Hydroxytryptamine -

Screening of 300 Drugs in Blood Utilizing Second Generation
Forensic Screening of 300 Drugs in Blood Utilizing Exactive Plus High-Resolution Accurate Mass Spectrometer and ExactFinder Software Kristine Van Natta, Marta Kozak, Xiang He Forensic Toxicology use Only Drugs analyzed Compound Compound Compound Atazanavir Efavirenz Pyrilamine Chlorpropamide Haloperidol Tolbutamide 1-(3-Chlorophenyl)piperazine Des(2-hydroxyethyl)opipramol Pentazocine Atenolol EMDP Quinidine Chlorprothixene Hydrocodone Tramadol 10-hydroxycarbazepine Desalkylflurazepam Perimetazine Atropine Ephedrine Quinine Cilazapril Hydromorphone Trazodone 5-(p-Methylphenyl)-5-phenylhydantoin Desipramine Phenacetin Benperidol Escitalopram Quinupramine Cinchonine Hydroquinine Triazolam 6-Acetylcodeine Desmethylcitalopram Phenazone Benzoylecgonine Esmolol Ranitidine Cinnarizine Hydroxychloroquine Trifluoperazine Bepridil Estazolam Reserpine 6-Monoacetylmorphine Desmethylcitalopram Phencyclidine Cisapride HydroxyItraconazole Trifluperidol Betaxolol Ethyl Loflazepate Risperidone 7(2,3dihydroxypropyl)Theophylline Desmethylclozapine Phenylbutazone Clenbuterol Hydroxyzine Triflupromazine Bezafibrate Ethylamphetamine Ritonavir 7-Aminoclonazepam Desmethyldoxepin Pholcodine Clobazam Ibogaine Trihexyphenidyl Biperiden Etifoxine Ropivacaine 7-Aminoflunitrazepam Desmethylmirtazapine Pimozide Clofibrate Imatinib Trimeprazine Bisoprolol Etodolac Rufinamide 9-hydroxy-risperidone Desmethylnefopam Pindolol Clomethiazole Imipramine Trimetazidine Bromazepam Felbamate Secobarbital Clomipramine Indalpine Trimethoprim Acepromazine Desmethyltramadol Pipamperone -

CISAPRIDE (Veterinary—Systemic)
CISAPRIDE (Veterinary—Systemic) There are no human- or veterinary-labeled commercial ELUS,CANDelayed gastric emptying (treatment)EL; or cisapride products in the United States or Canada. ELUS,CANSmall bowel motility disorders (treatment)EL— Although no feline studies are available, the Category: Gastrointestinal emptying adjunct; documented effects of cisapride in healthy dogs and peristaltic stimulant. other animals suggest it may have efficacy in disorders that benefit from stimulation of gastric, Indications small intestinal, or colonic motility and from Note: Cisapride is not specifically approved for shortened transit time in cats (Evidence rating: B- {R-2; 4-7; 21-24} veterinary use. In other USP information 2). monographs, the ELUS and ELCAN designations refer to uses that are not included in U.S. and Canadian Dogs product labeling; however, in this monograph Potentially effective they reflect the lack of commercial product ELUS,CANGastroesophageal reflux (treatment)EL; availability in the countries indicated. See also the ELUS,CANDelayed gastric emptying (treatment)EL; Regulatory Considerations section below in this ELUS,CANSmall bowel motility disorders (treatment)EL; or monograph. US,CAN EL Colonic motility disorders (treatment)EL — Classification as Accepted, Potentially effective, Although no studies of clinical disease states are or Unaccepted is an evaluation of reasonable use available, studies of the effects of cisapride in that considers clinical circumstances, including healthy dogs suggest it may have efficacy in the availability of other therapies. The quality of disorders that benefit from stimulation of gastric, evidence reviewed for an indication is shown by small intestinal, or colonic motility and from the evidence rating. shortened transit time (Evidence rating: B-2 [table 1][table 2][table3]).{R-4-7; 17; 21-24} Cats Accepted Note: There is no evidence that cisapride is effective in ELUS,CAN EL Constipation, chronic (treatment) ; or the treatment of megaesophagus in dogs. -

Perphenazine-151548-PI.Pdf
PRESCRIBING INFORMATION PERPHENAZINE Perphenazine Tablets USP 2 mg , 4 mg, 8mg, and 16 mg Antipsychotic/Antiemetic AA Pharma Inc. DATE OF REVISION: April 12, 2012 1165 Creditsone Road, Unit #1 Vaughan, Ontario L4K 4N7 Control Number: 151548 PRESCRIBING INFORMATION PERPHENAZINE Perphenazine Tablets USP 2 mg, 4 mg, 8 mg and 16 mg THERAPEUTIC CLASSIFICATION Antipsychotic/Antiemetic ACTIONS AND CLINICAL PHARMACOLOGY Perphenazine is a piperazine phenothiazine derivative with antipsychotic, antiemetic and weak sedative activity. Perphenazine has actions similar to those of other phenothiazine derivatives but appears to be less sedating and to have a weak propensity for causing hypotension or potentiating the effects of CNS depressants and anesthetics. However, it produces a high incidence of extrapyramidal reactions. Perphenazine is well absorbed from the gastrointestinal tract. Onset of action following oral administration is 30 to 40 minutes. Duration of action is 3 to 4 hours. Perphenazine distributes to most body tissues with high concentrations being distributed into liver and spleen. Perphenazine enters the enterohepatic circulation and is excreted chiefly in the feces. INDICATIONS AND CLINICAL USE Perphenazine is indicated in the management of manifestations of psychotic disorders. It is also effective in controlling nausea and vomiting due to stimulation of the chemoreceptor trigger zone. 1 Perphenazine has not been shown effective for the management of behavioral complications in patients with mental retardation. CONTRAINDICATIONS Should not be administered in the presence of circulatory collapse, altered states of consciousness or comatose states, particularly when these are due to intoxication with central depressant drugs (alcohol, hypnotics, narcotics). It is contraindicated in severely depressed patients, in the presence of blood dyscrasias, liver disease, renal insufficiency, pheochromocytoma, or in patients with severe cardiovascular disorders or a history of hypersensitivity to phenothiazine derivatives. -

Neuromodulators for Functional Gastrointestinal Disorders (Disorders of Gutlbrain Interaction): a Rome Foundation Working Team Report Douglas A
Gastroenterology 2018;154:1140–1171 SPECIAL REPORT Neuromodulators for Functional Gastrointestinal Disorders (Disorders of GutLBrain Interaction): A Rome Foundation Working Team Report Douglas A. Drossman,1,2 Jan Tack,3 Alexander C. Ford,4,5 Eva Szigethy,6 Hans Törnblom,7 and Lukas Van Oudenhove8 1Center for Functional Gastrointestinal and Motility Disorders, University of North Carolina, Chapel Hill, North Carolina; 2Center for Education and Practice of Biopsychosocial Care and Drossman Gastroenterology, Chapel Hill, North Carolina; 3Translational Research Center for Gastrointestinal Disorders, University of Leuven, Leuven, Belgium; 4Leeds Institute of Biomedical and Clinical Sciences, University of Leeds, Leeds, United Kingdom; 5Leeds Gastroenterology Institute, St James’s University Hospital, Leeds, United Kingdom; 6Departments of Psychiatry and Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania; 7Departments of Internal Medicine and Clinical Nutrition, Institute of Medicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden; and 8Laboratory for BrainÀGut Axis Studies, Translational Research Center for Gastrointestinal Disorders, University of Leuven, Leuven, Belgium BACKGROUND & AIMS: Central neuromodulators (antide- summary information and guidelines for the use of central pressants, antipsychotics, and other central nervous neuromodulators in the treatment of chronic gastrointestinal systemÀtargeted medications) are increasingly used for treat- symptoms and FGIDs. Further studies are needed to confirm ment -

Cisapride: a Novel Gastroprokinetic Drug V
The Canadian Journal of Hospital Pharmacy - Volume 44, No. 4, August, 1991 175 Cisapride: A Novel Gastroprokinetic Drug V. Arora and M. Spino ABSTRACT RESUME Impaired gastrointestinal motility underlies a multitude of Une motilite gastro-intestinale pathologique est le signe digestive complaints. Metoclopramide, an antidopaminer d 'une multitude de maladies digestives. Le metoclopramide, gic and cholinomimetic agen~ was the first prokinetic drug un agent anti-dopaminergique et cholinomimetique, fut le used to treat such conditions, but a high incidence of premier medicament procinetique utilise pour traiter de adverse effects has limited its use, especially in infants. tel/es conditions mais une frequence elevee des reactions Domperidone, the second prokinetic drug marketed in indesirables a limite son usage, surtout chez !es nourrissons. Canada, is a potent peripheral dopamine receptor an Le domperidone, le deuxieme medicament procinetique tagonist which does not cross the blood-brain barrier well commercialise au Canada, est un antagoniste puissant des and, therefore, displays minimal CNS side effects. Cisapride recepteurs dopaminergiques peripheriques qui ne traverse is a gastroprokinetic agent which appears to act mainly pas beaucoup la barriere hemo-encephalique et done, by releasing acetylcholine from the myenteric plexus of provoque peu d'effets secondaires au systeme nerveux the gut. It has no dopamine-blocking activity, and does central (SNC). Le cisapride est un nouvel agent gastro not share the serious CNS side effects of other drugs in procinetique qui semble agir principalement par la libe its class. These drugs stimulate gastric and small intestinal ration de l'acetylcholine du plexus myenterique de l'intestin. activity, but cisapride also enhances colonic motility. -

Understanding Drug Interactions in Dental Practice
2019 Understanding Drug Interactions in Dental Practice Money Makes the World Go Round, But Drugs Can Make it Spin! Dr. Mark Donaldson, BSP, RPH, ACPR, PHARMD, FASHP, FACHE Associate Vice President Vizient Pharmacy Advisory Solutions [email protected] Our Clinician: Dr. Mark Donaldson BSP, RPH, PHARMD, FASHP, FACHE received his baccalaureate degree from the University of British Columbia and his Doctorate in Clinical Pharmacy from the University of Washington. He completed a residency at Vancouver General Hospital, and has practiced as a clinical pharmacy specialist, clinical coordinator and director of pharmacy services at many healthcare organizations in both Canada and the United States. He is currently the Associate Vice President of Clinical Pharmacy for Vizient’s Advisory Solutions, and lives in Whitefish, Montana. Dr. Donaldson is a Clinical Professor in the Department of Pharmacy at the University of Montana in Missoula, Clinical Associate Professor in the School of Dentistry at the Oregon Health & Sciences University in Portland, Oregon, and affiliate faculty in the School of Dentistry at UBC. He has a special interest in dental pharmacology and has lectured internationally to both dental and medical practitioners. He has spent the last 20 years focusing on dental pharmacology and dental therapeutics, and is a leader in the field. Dr. Donaldson has published numerous peer-reviewed works and textbook chapters. He currently serves on the Editorial Board for the Journal Healthcare Executive and the Journal of the American Dental Association and is a reviewer for over ten other different journals. He is board certified in healthcare management and is the Past- President and current Regent of the American College of Healthcare Executives’ Montana Chapter. -

DOMASYS Standard
New Zealand Data Sheet 04 December 2018 Neulactil-periciazine DATA SHEET 1 NEULACTIL TABLETS Neulactil 2.5 mg tablets Neulactil 10 mg tablets 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Periciazine 2.5mg Periciazine 10mg Excipients with known effect: lactose monohydrate and wheat starch For the full list of excipients, see section 6.1. 3 PHARMACEUTICAL FORM Neulactil tablets 2.5 mg: yellow, scored, marked NEULACTIL 10 mg: yellow, scored, marked 10 4 CLINICAL PARTICULARS 4.1 THERAPEUTIC INDICATIONS Periciazine is indicated: 1. In adults with schizophrenia or other psychoses, for the treatment of symptoms or prevention of relapse. 2. In anxiety, psychomotor agitation, violent or dangerously impulsive behaviour. periciazine is used as an adjunct to the short-term management of these conditions. Periciazine tablets are not recommended for children (see Section 4.3 and 4.4). neulactil-ccsiv4-dsv11-04dec18 Page 1 New Zealand Data Sheet 04 December 2018 Neulactil-periciazine 4.2 DOSE AND METHOD OF ADMINISTRATION Dosage requirements vary with the individual and the severity of the condition being treated. Initial dosage should be low with progressive increases until the desired response is obtained, after which dosage should be adjusted to maintain control of symptoms. Severe conditions (Indication 1) Adults Initially 75 mg per day in divided doses. Dosage should be increased by 25 mg per day at weekly intervals until optimum effect is achieved. Maintenance therapy would not normally be expected to exceed 300mg per day. Elderly Initially 15-30 mg per day in divided doses. If this is well tolerated the dosage may be increased if necessary for optimum control of behaviour. -
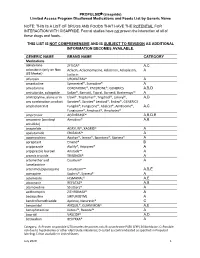
PROPULSID® (Cisapride) Limited Access Program
PROPULSID (cisapride) Limited Access Program Disallowed Medications and Foods List by Generic Name NOTE: THIS IS A LIST OF DRUGS AND FOODS THAT HAVE THE POTENTIAL FOR INTERACTION WITH CISAPRIDE. Formal studies have not proven the interaction of all of these drugs and foods. THIS LIST IS NOT COMPREHENSIVE AND IS SUBJECT TO REVISION AS ADDITIONAL INFORMATION BECOMES AVAILABLE. GENERIC NAME BRAND NAME CATEGORY Medications abiraterone ZYTIGA® A,C aclarubicin (only on Non Aclacin, Aclacinomycine, Aclacinon, Aclaplastin, A US Market) Jaclacin alfuzosin UROXATRAL® A amantadine Symmetrel®, Symadine® A amiodarone CORDARONE®, PACERONE®, GENERICS A,B,D amisulpride, sultopride Solian®, Barnotil, Topral, Barnetil, Barhemsys™ A amitriptyline, alone or in Elavil®, Tryptomer®, Tryptizol®, Laroxyl®, A,D any combination product Saroten®, Sarotex® Lentizol®, Endep®, GENERICS amphotericin B Fungilin®, Fungizone®, Abelcet®, AmBisome®, A,C Fungisome®, Amphocil®, Amphotec® amprenavir AGENERASE® A,B,D,E amsacrine (acridinyl Amsidine® A,B anisidide) anagrelide AGRYLIN®, XAGRID® A apalutamide ERLEADA™ A apomorphine Apokyn®, Ixense®, Spontane®, Uprima® A aprepitant Emend® B aripiprazole Abilify®, Aripiprex® A aripiprazole lauroxil Aristada™ A arsenic trioxide TRISENOX® A artemether and Coartem® A lumefantrine artenimol/piperaquine Eurartesim™ A,B,E asenapine Saphris®, Sycrest® A astemizole HISMANAL® A,E atazanavir REYATAZ® A,B atomoxetine Strattera® A azithromycin ZITHROMAX® A bedaquiline SIRTURO(TM) A bendroflumethiazide Aprinox, Naturetin® C benperidol ANQUIL®,