Structural and Functional Analysis of PTPMT1, a Phosphatase Required for Cardiolipin Synthesis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Supplementary Table S1. Upregulated Genes Differentially
Supplementary Table S1. Upregulated genes differentially expressed in athletes (p < 0.05 and 1.3-fold change) Gene Symbol p Value Fold Change 221051_s_at NMRK2 0.01 2.38 236518_at CCDC183 0.00 2.05 218804_at ANO1 0.00 2.05 234675_x_at 0.01 2.02 207076_s_at ASS1 0.00 1.85 209135_at ASPH 0.02 1.81 228434_at BTNL9 0.03 1.81 229985_at BTNL9 0.01 1.79 215795_at MYH7B 0.01 1.78 217979_at TSPAN13 0.01 1.77 230992_at BTNL9 0.01 1.75 226884_at LRRN1 0.03 1.74 220039_s_at CDKAL1 0.01 1.73 236520_at 0.02 1.72 219895_at TMEM255A 0.04 1.72 201030_x_at LDHB 0.00 1.69 233824_at 0.00 1.69 232257_s_at 0.05 1.67 236359_at SCN4B 0.04 1.64 242868_at 0.00 1.63 1557286_at 0.01 1.63 202780_at OXCT1 0.01 1.63 1556542_a_at 0.04 1.63 209992_at PFKFB2 0.04 1.63 205247_at NOTCH4 0.01 1.62 1554182_at TRIM73///TRIM74 0.00 1.61 232892_at MIR1-1HG 0.02 1.61 204726_at CDH13 0.01 1.6 1561167_at 0.01 1.6 1565821_at 0.01 1.6 210169_at SEC14L5 0.01 1.6 236963_at 0.02 1.6 1552880_at SEC16B 0.02 1.6 235228_at CCDC85A 0.02 1.6 1568623_a_at SLC35E4 0.00 1.59 204844_at ENPEP 0.00 1.59 1552256_a_at SCARB1 0.02 1.59 1557283_a_at ZNF519 0.02 1.59 1557293_at LINC00969 0.03 1.59 231644_at 0.01 1.58 228115_at GAREM1 0.01 1.58 223687_s_at LY6K 0.02 1.58 231779_at IRAK2 0.03 1.58 243332_at LOC105379610 0.04 1.58 232118_at 0.01 1.57 203423_at RBP1 0.02 1.57 AMY1A///AMY1B///AMY1C///AMY2A///AMY2B// 208498_s_at 0.03 1.57 /AMYP1 237154_at LOC101930114 0.00 1.56 1559691_at 0.01 1.56 243481_at RHOJ 0.03 1.56 238834_at MYLK3 0.01 1.55 213438_at NFASC 0.02 1.55 242290_at TACC1 0.04 1.55 ANKRD20A1///ANKRD20A12P///ANKRD20A2/// -

RNA Epigenetics: Fine-Tuning Chromatin Plasticity and Transcriptional Regulation, and the Implications in Human Diseases
G C A T T A C G G C A T genes Review RNA Epigenetics: Fine-Tuning Chromatin Plasticity and Transcriptional Regulation, and the Implications in Human Diseases Amber Willbanks, Shaun Wood and Jason X. Cheng * Department of Pathology, Hematopathology Section, University of Chicago, Chicago, IL 60637, USA; [email protected] (A.W.); [email protected] (S.W.) * Correspondence: [email protected] Abstract: Chromatin structure plays an essential role in eukaryotic gene expression and cell identity. Traditionally, DNA and histone modifications have been the focus of chromatin regulation; however, recent molecular and imaging studies have revealed an intimate connection between RNA epigenetics and chromatin structure. Accumulating evidence suggests that RNA serves as the interplay between chromatin and the transcription and splicing machineries within the cell. Additionally, epigenetic modifications of nascent RNAs fine-tune these interactions to regulate gene expression at the co- and post-transcriptional levels in normal cell development and human diseases. This review will provide an overview of recent advances in the emerging field of RNA epigenetics, specifically the role of RNA modifications and RNA modifying proteins in chromatin remodeling, transcription activation and RNA processing, as well as translational implications in human diseases. Keywords: 5’ cap (5’ cap); 7-methylguanosine (m7G); R-loops; N6-methyladenosine (m6A); RNA editing; A-to-I; C-to-U; 2’-O-methylation (Nm); 5-methylcytosine (m5C); NOL1/NOP2/sun domain Citation: Willbanks, A.; Wood, S.; (NSUN); MYC Cheng, J.X. RNA Epigenetics: Fine-Tuning Chromatin Plasticity and Transcriptional Regulation, and the Implications in Human Diseases. Genes 2021, 12, 627. -

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

Pharmacological Targeting of the Mitochondrial Phosphatase PTPMT1 by Dahlia Doughty Shenton Department of Biochemistry Duke
Pharmacological Targeting of the Mitochondrial Phosphatase PTPMT1 by Dahlia Doughty Shenton Department of Biochemistry Duke University Date: May 1 st 2009 Approved: ___________________________ Dr. Patrick J. Casey, Supervisor ___________________________ Dr. Perry J. Blackshear ___________________________ Dr. Anthony R. Means ___________________________ Dr. Christopher B. Newgard ___________________________ Dr. John D. York Dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Department of Biochemistry in the Graduate School of Duke University 2009 ABSTRACT Pharmacological Targeting of the Mitochondrial Phosphatase PTPMT1 by Dahlia Doughty Shenton Department of Biochemistry Duke University Date: May 1 st 2009 Approved: ___________________________ Dr. Patrick J. Casey, Supervisor ___________________________ Dr. Perry J. Blackshear ___________________________ Dr. Anthony R. Means ___________________________ Dr. Christopher B. Newgard ___________________________ Dr. John D. York An abstract of a dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Department of Biochemistry in the Graduate School of Duke University 2009 Copyright by Dahlia Doughty Shenton 2009 Abstract The dual specificity protein tyrosine phosphatases comprise the largest and most diverse group of protein tyrosine phosphatases and play integral roles in the regulation of cell signaling events. The dual specificity protein tyrosine phosphatases impact multiple -

Structural and Functional Analysis of PTPMT1, a Phosphatase Required for Cardiolipin Synthesis
Structural and functional analysis of PTPMT1, a phosphatase required for cardiolipin synthesis Junyu Xiaoa,1, James L. Engela,1, Ji Zhanga, Mark J. Chenb, Gerard Manningb, and Jack E. Dixona,c,d,e,2 aDepartment of Pharmacology, University of California, La Jolla, CA 92093; bRazavi Newman Center for Bioinformatics, Salk Institute, La Jolla, CA 92037; cDepartment of Cellular and Molecular Medicine, University of California, La Jolla, CA 92093; dDepartment of Chemistry and Biochemistry, University of California, La Jolla, CA 92093; and eHoward Hughes Medical Institute, Chevy Chase, MD 20815 Contributed by Jack E. Dixon, June 9, 2011 (sent for review December 29, 2010) PTPMT1 (PTP localized to the Mitochondrion 1) is a member of the chondria, and its level is not affected by the loss of PTPMT1, sug- protein tyrosine phosphatase superfamily that is localized exclu- gesting that PI(5)P is not its endogenous substrate (10). We sively to the mitochondrion. We recently reported that PTPMT1 recently demonstrated that the physiological target of PTPMT1 dephosphorylates phosphatidylglycerol phosphate, an essential is phosphatidylglycerol phosphate (PGP) (11), which is structu- intermediate of cardiolipin biosynthesis. To gain further insights rally remarkably similar to PI(5)P (Fig. 1C). into the molecular basis of PTPMT1 function, we determined the Phosphatidylglycerol (PG), the product of PTPMT1’s activity, crystal structures of the phosphatase domain of PTPMT1. PTPMT1 is an essential component of pulmonary surfactant, and a precur- exhibits a canonical protein tyrosine phosphatase domain fold, sor for cardiolipin biosynthesis (Fig. 1D and Fig. S2). Cardiolipin resembling many dual-specificity phosphatases such as phospha- is a glycerophospholipid found predominantly in the mitochon- tase and tensin homolog and vaccinia H1-related phosphatase. -

Revealing the Action Mechanisms of Dexamethasone on the Birth Weight
® Observational Study Medicine OPEN Revealing the action mechanisms of dexamethasone on the birth weight of infant using RNA-sequencing data of trophoblast cells ∗ Hongkai Shang, MDa, Liping Sun, BMa, Thorsten Braun, MDb, Qi Si, BMa, Jinyi Tong, MMa, Abstract Dexamethasone (DEX) could induce low birth weight of infant, and low birth weight has close associations with glucocorticoid levels, insulin resistance, hypertension, and metabolic syndrome in adulthood. This study was designed to reveal the action mechanisms of DEX on the birth weight of infant. Using quantitative real-time polymerase chain reaction (qRT-PCR), trophoblast cells of human placenta were identified and the optimum treatment time of DEX were determined. Trophoblast cells were treated by DEX (DEX group) or ethanol (control group) (each group had 3 samples), and then were performed with RNA-sequencing. Afterward, the differentially expressed genes (DEGs) were identified by R package, and their potential functions were successively enriched using DAVID database and Enrichr method. Followed by protein–protein interaction (PPI) network was constructed using Cytoscape software. Using Enrichr method and TargetScan software, the transcription factors (TFs) and micorRNAs (miRNAs) targeted the DEGs separately were predicted. Based on MsigDB database, gene set enrichment analysis (GSEA) was performed. There were 391 DEGs screened from the DEX group. Upregulated SRR and potassium voltage-gated channel subfamily J member 4(KCNJ4) and downregulated GALNT1 separately were enriched in PDZ (an acronym of PSD-95, Dlg, and ZO-1) domain binding and Mucin type O-glycan biosynthesis. In the PPI network, CDK2 and CDK4 had higher degrees. TFs ATF2 and E2F4 and miRNA miR-16 were predicted for the DEGs. -

Live-Cell Imaging Rnai Screen Identifies PP2A–B55α and Importin-Β1 As Key Mitotic Exit Regulators in Human Cells
LETTERS Live-cell imaging RNAi screen identifies PP2A–B55α and importin-β1 as key mitotic exit regulators in human cells Michael H. A. Schmitz1,2,3, Michael Held1,2, Veerle Janssens4, James R. A. Hutchins5, Otto Hudecz6, Elitsa Ivanova4, Jozef Goris4, Laura Trinkle-Mulcahy7, Angus I. Lamond8, Ina Poser9, Anthony A. Hyman9, Karl Mechtler5,6, Jan-Michael Peters5 and Daniel W. Gerlich1,2,10 When vertebrate cells exit mitosis various cellular structures can contribute to Cdk1 substrate dephosphorylation during vertebrate are re-organized to build functional interphase cells1. This mitotic exit, whereas Ca2+-triggered mitotic exit in cytostatic-factor- depends on Cdk1 (cyclin dependent kinase 1) inactivation arrested egg extracts depends on calcineurin12,13. Early genetic studies in and subsequent dephosphorylation of its substrates2–4. Drosophila melanogaster 14,15 and Aspergillus nidulans16 reported defects Members of the protein phosphatase 1 and 2A (PP1 and in late mitosis of PP1 and PP2A mutants. However, the assays used in PP2A) families can dephosphorylate Cdk1 substrates in these studies were not specific for mitotic exit because they scored pro- biochemical extracts during mitotic exit5,6, but how this relates metaphase arrest or anaphase chromosome bridges, which can result to postmitotic reassembly of interphase structures in intact from defects in early mitosis. cells is not known. Here, we use a live-cell imaging assay and Intracellular targeting of Ser/Thr phosphatase complexes to specific RNAi knockdown to screen a genome-wide library of protein substrates is mediated by a diverse range of regulatory and targeting phosphatases for mitotic exit functions in human cells. We subunits that associate with a small group of catalytic subunits3,4,17. -

Phosphatases Page 1
Phosphatases esiRNA ID Gene Name Gene Description Ensembl ID HU-05948-1 ACP1 acid phosphatase 1, soluble ENSG00000143727 HU-01870-1 ACP2 acid phosphatase 2, lysosomal ENSG00000134575 HU-05292-1 ACP5 acid phosphatase 5, tartrate resistant ENSG00000102575 HU-02655-1 ACP6 acid phosphatase 6, lysophosphatidic ENSG00000162836 HU-13465-1 ACPL2 acid phosphatase-like 2 ENSG00000155893 HU-06716-1 ACPP acid phosphatase, prostate ENSG00000014257 HU-15218-1 ACPT acid phosphatase, testicular ENSG00000142513 HU-09496-1 ACYP1 acylphosphatase 1, erythrocyte (common) type ENSG00000119640 HU-04746-1 ALPL alkaline phosphatase, liver ENSG00000162551 HU-14729-1 ALPP alkaline phosphatase, placental ENSG00000163283 HU-14729-1 ALPP alkaline phosphatase, placental ENSG00000163283 HU-14729-1 ALPPL2 alkaline phosphatase, placental-like 2 ENSG00000163286 HU-07767-1 BPGM 2,3-bisphosphoglycerate mutase ENSG00000172331 HU-06476-1 BPNT1 3'(2'), 5'-bisphosphate nucleotidase 1 ENSG00000162813 HU-09086-1 CANT1 calcium activated nucleotidase 1 ENSG00000171302 HU-03115-1 CCDC155 coiled-coil domain containing 155 ENSG00000161609 HU-09022-1 CDC14A CDC14 cell division cycle 14 homolog A (S. cerevisiae) ENSG00000079335 HU-11533-1 CDC14B CDC14 cell division cycle 14 homolog B (S. cerevisiae) ENSG00000081377 HU-06323-1 CDC25A cell division cycle 25 homolog A (S. pombe) ENSG00000164045 HU-07288-1 CDC25B cell division cycle 25 homolog B (S. pombe) ENSG00000101224 HU-06033-1 CDKN3 cyclin-dependent kinase inhibitor 3 ENSG00000100526 HU-02274-1 CTDSP1 CTD (carboxy-terminal domain, -

Dual Specificity Phosphatases from Molecular Mechanisms to Biological Function
International Journal of Molecular Sciences Dual Specificity Phosphatases From Molecular Mechanisms to Biological Function Edited by Rafael Pulido and Roland Lang Printed Edition of the Special Issue Published in International Journal of Molecular Sciences www.mdpi.com/journal/ijms Dual Specificity Phosphatases Dual Specificity Phosphatases From Molecular Mechanisms to Biological Function Special Issue Editors Rafael Pulido Roland Lang MDPI • Basel • Beijing • Wuhan • Barcelona • Belgrade Special Issue Editors Rafael Pulido Roland Lang Biocruces Health Research Institute University Hospital Erlangen Spain Germany Editorial Office MDPI St. Alban-Anlage 66 4052 Basel, Switzerland This is a reprint of articles from the Special Issue published online in the open access journal International Journal of Molecular Sciences (ISSN 1422-0067) from 2018 to 2019 (available at: https: //www.mdpi.com/journal/ijms/special issues/DUSPs). For citation purposes, cite each article independently as indicated on the article page online and as indicated below: LastName, A.A.; LastName, B.B.; LastName, C.C. Article Title. Journal Name Year, Article Number, Page Range. ISBN 978-3-03921-688-8 (Pbk) ISBN 978-3-03921-689-5 (PDF) c 2019 by the authors. Articles in this book are Open Access and distributed under the Creative Commons Attribution (CC BY) license, which allows users to download, copy and build upon published articles, as long as the author and publisher are properly credited, which ensures maximum dissemination and a wider impact of our publications. The book as a whole is distributed by MDPI under the terms and conditions of the Creative Commons license CC BY-NC-ND. Contents About the Special Issue Editors .................................... -
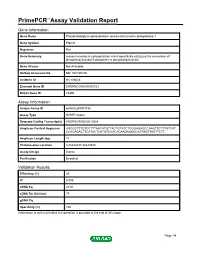
Primepcr™Assay Validation Report
PrimePCR™Assay Validation Report Gene Information Gene Name Phosphatidylglycerophosphatase and protein-tyrosine phosphatase 1 Gene Symbol Ptpmt1 Organism Rat Gene Summary mouse homolog is a phosphatase which specifically catalyzes the conversion of phosphatidylinositol 5-phosphate to phosphatidylinositol Gene Aliases Not Available RefSeq Accession No. NM_001105726 UniGene ID Rn.108023 Ensembl Gene ID ENSRNOG00000009723 Entrez Gene ID 29390 Assay Information Unique Assay ID qRnoCED0003185 Assay Type SYBR® Green Detected Coding Transcript(s) ENSRNOT00000013584 Amplicon Context Sequence AGCGCTTTGTCCTTTAACATGTTACTGTATCTCGGAGAGCCAAGTTCTTTGTCAT CCACAGACTTCATACTCATGTCAGCACAAGAGACCAGTAGTTAGTTCTT Amplicon Length (bp) 74 Chromosome Location 3:86444437-86444540 Assay Design Exonic Purification Desalted Validation Results Efficiency (%) 99 R2 0.999 cDNA Cq 20.91 cDNA Tm (Celsius) 79 gDNA Cq Specificity (%) 100 Information to assist with data interpretation is provided at the end of this report. Page 1/4 PrimePCR™Assay Validation Report Ptpmt1, Rat Amplification Plot Amplification of cDNA generated from 25 ng of universal reference RNA Melt Peak Melt curve analysis of above amplification Standard Curve Standard curve generated using 20 million copies of template diluted 10-fold to 20 copies Page 2/4 PrimePCR™Assay Validation Report Products used to generate validation data Real-Time PCR Instrument CFX384 Real-Time PCR Detection System Reverse Transcription Reagent iScript™ Advanced cDNA Synthesis Kit for RT-qPCR Real-Time PCR Supermix SsoAdvanced™ SYBR® Green Supermix Experimental Sample qPCR Reference Total RNA Data Interpretation Unique Assay ID This is a unique identifier that can be used to identify the assay in the literature and online. Detected Coding Transcript(s) This is a list of the Ensembl transcript ID(s) that this assay will detect. -

Identification of Potential Core Genes in Sevoflurance Induced Myocardial
Identication of Potential core genes in Sevourance induced Myocardial Energy Metabolism in Patients Undergoing Off-pump Coronary Artery Bypass Graft Surgery using Bioinformatics analysis Hua Lin ( [email protected] ) Tianjin Medical University General Hospital Airport Site Research article Keywords: sevourane, Myocardial Energy Metabolism, Off-pump Coronary Artery Bypass Graft Surgery Posted Date: November 18th, 2019 DOI: https://doi.org/10.21203/rs.2.17434/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/15 Abstract Background: Myocardial ischemia-reperfusion injury always happened after Off-pump coronary artery bypass graft(OPCABG), and this can not be avoided altogether. In this study, we tried to detect potential genes of sevourane-induced myocardial energy metabolism in patients undergoing OPCABG using bioinformatics analysis. Methods: We download and analyze the gene expression prole data from the Gene Expression Omnibus(GEO) database using bioinformatics methods. We downloded the gene expression data from the Gene Expression Omnibus(GEO) database using bioinformatics methods. Gene Ontology(GO) functional annotation analysis and Kyoto Encyclopedia of Genes and Genomes(KEGG) pathway enrichment analysis were used to analysis the screened differentially expressed genes(DEGs). Then, we established a protein–protein interaction (PPI) network to nd hub genes associated with myocardial energy metabolism. Results: Through PPI network, we nd ten hub genes, including JUN, EGR1, ATF3, FOSB, JUNB, DUSP1, EGR2, NR4A1, BTG2, NR4A2. Conclusions: In conclusion, the proteins encoded by EGR1ATF3c-FosBtg2JunBDUSP1NR4A1BTG2 and NR4A2 were related to cardiac function. ATF3, FOSB, JUNB, DUSP1, NR4A1, NR4A2 are related to apoptosis of cardiomyocytes. The protein encoded by BTG2 is related to hypertrophy. -

Comp Add 1.Pdf
hESC exp1 hESC exp2 hESC exp3 hESC exp4hESC exp5hESC exp6 hESC exp7 hESC exp8 hESC exp9 ACTA1 CDC25A POU5F1 LUM ARID1B BRIX1 ORC1 POU5F1 CHEK2 ACTC1 CYP26A1 TDGF1 COL3A1 ARID5B CD9 PAK1 SOX2 CKS1B ACTN3 DNMT3A DPPA4 COL1A1 BHLHE40 COMMD3 IGF2 NANOG CCNB1 ADD2 DTYMK LIN28A IGFBP3 BTF3 CRABP2 C14orf156 HESX1 PODXL PARP1 EPHA1 NANOG ACTA2 CCDC6 DIAPH2 PHC1 ZFP42 TDGF1 ALPL ETV4 DNMT3B P4HA2 CDX2 DNMT3B SPARC ZSCAN10 TMSB15A AMD1 GPR19 TERF1 SPARC CEBPZ LEFTY2 NTHL1 TGIF1 PFN1 BIRC5 LPAR4 SEMA6A COL1A2 CNOT3 EDNRB RUVBL1 DNMT3A CFL1 ATP1A2 HELLS M6PR KRT18 COMMD3 FGF4 TTC3 DNMT3B DNMT3B BMPR1A MYBL2 SNRPN DCN CTCF FGF5 IGFBP3 LIN28A PCNA BUB1 ORC1 L1TD1 BMP4 CUX1 FOXD3 FAM89B NPM1 TERF1 BUB1B ORC2 LEFTY1 COL5A1 DBP GABRB3 PPAT FAS UTP18 C1QBP POU5F1 GAL COL2A1 DEK GAL ARHGDIB TDGF1 GDF3 CASP3 PWP2 SEPHS1 RHOBTB3 DLX1 GATA6 POLG2 GDF3 VCP CBS RBM4 GABRB3 NDRG1 E2F1 GBX2 TRIM37 FLJ21837 GJA1 CCNA2 TDGF1 SOX2 COL6A3 E2F2 GDF3 RNF138 DPPA4 CLDN6 CCNB1 ZIC3 LECT1 CST3 E2F3 GRB7 MTHFD1 HMGA1 NPM1 CDK1 HESX1 ANGEL2 KRT8 E2F4 IFITM2 TERF2IP ERH NCL CDC6 SLC5A6 BUB1 CD47 EBF1 IL6ST TDP1 CKS1B FAS CDC20 CLDN6 PSIP1 HAND1 EBF2 IGF2BP2 JARID2 CHEK2 SFRP1 CDC25A RRP9 IDO1 IGF2 EBF3 KIT ARL6IP5 CLDN6 FZD7 CTSC GDF3 HELLS CXCL14 EBF4 LEFTY1 LAS1L GJA1 Pou5f1 CHEK1 GJC1 GPC4 COL11A1 EOMES LIFR CEBPD ERH CRABP1 CHEK2 ITGB1BP3 IGFBP7 ERH LIN28A CTSB SOX2 CRABP2 WSCD1 CYP26A1 IL6ST Esrrb Nanog SEPHS1 LIN28A CRMP1 NCAPH MCM5 COL5A2 ESX1l NODAL DFNA5 HMGA1 CSE1L PRKD3 MTHFD1 KRT7 ETS1 NOG LUM ZFP42 CXADR GOLGA7 PPAT KRT19 ETS2 NR5A2 LCK CYP26A1 L1TD1 SLC16A1 BMP1