Facet Joint Nerve Injections Product Applicability
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Vertebral Column and Thorax
Introduction to Human Osteology Chapter 4: Vertebral Column and Thorax Roberta Hall Kenneth Beals Holm Neumann Georg Neumann Gwyn Madden Revised in 1978, 1984, and 2008 The Vertebral Column and Thorax Sternum Manubrium – bone that is trapezoidal in shape, makes up the superior aspect of the sternum. Jugular notch – concave notches on either side of the superior aspect of the manubrium, for articulation with the clavicles. Corpus or body – flat, rectangular bone making up the major portion of the sternum. The lateral aspects contain the notches for the true ribs, called the costal notches. Xiphoid process – variably shaped bone found at the inferior aspect of the corpus. Process may fuse late in life to the corpus. Clavicle Sternal end – rounded end, articulates with manubrium. Acromial end – flat end, articulates with scapula. Conoid tuberosity – muscle attachment located on the inferior aspect of the shaft, pointing posteriorly. Ribs Scapulae Head Ventral surface Neck Dorsal surface Tubercle Spine Shaft Coracoid process Costal groove Acromion Glenoid fossa Axillary margin Medial angle Vertebral margin Manubrium. Left anterior aspect, right posterior aspect. Sternum and Xyphoid Process. Left anterior aspect, right posterior aspect. Clavicle. Left side. Top superior and bottom inferior. First Rib. Left superior and right inferior. Second Rib. Left inferior and right superior. Typical Rib. Left inferior and right superior. Eleventh Rib. Left posterior view and left superior view. Twelfth Rib. Top shows anterior view and bottom shows posterior view. Scapula. Left side. Top anterior and bottom posterior. Scapula. Top lateral and bottom superior. Clavicle Sternum Scapula Ribs Vertebrae Body - Development of the vertebrae can be used in aging of individuals. -

National Imaging Associates, Inc. Clinical Guidelines FACET JOINT
National Imaging Associates, Inc. Clinical guidelines Original Date: July 1, 2015 FACET JOINT INJECTIONS OR BLOCKS Page 1 of 4 “FOR FLORIDA BLUE MEMBERS ONLY” CPT Codes: Last Review Date: May 28, 2015 Cervical/Thoracic Region: 64490 (+ 64491, +64492) Lumbar/Sacral Region: 64493 (+64494, +64495) Medical Coverage Guideline Number: Last Revised Date: 02-61000-30 Responsible Department: Implementation Date: July 2015 Clinical Operations “FOR FLORIDA BLUE MEMBERS ONLY” INTRODUCTION Facet joints (also called zygapophysial joints or z-joints), are posterior to the vertebral bodies in the spinal column and connect the vertebral bodies to each other. They are located at the junction of the inferior articular process of a more cephalad vertebra, and the superior articular process of a more caudal vertebra. These joints provide stability and enable movement, allowing the spine to bend, twist, and extend in different directions. They also restrict hyperextension and hyperflexion. Facet joints are clinically important spinal pain generators in those with chronic spinal pain. Facet joints may refer pain to adjacent structures, making the underlying diagnosis difficult, as referred pain may assume a pseudoradicular pattern. Lumbar facet joints may refer pain to the back, buttocks, and lower extremities while cervical facet joints may refer pain to the head, neck and shoulders. Imaging findings are of little value in determining the source and location of ‘facet joint syndrome’, a term referring to back pain caused by pathology at the facet joints. Imaging studies may detect changes in facet joint architecture, but correlation between radiologic findings and symptoms is unreliable. Although clinical signs are also unsuitable for diagnosing facet joint-mediated pain, they may be of value in selecting candidates for controlled local anesthetic blocks of either the medial branches or the facet joint itself. -

The Erector Spinae Plane Block a Novel Analgesic Technique in Thoracic Neuropathic Pain
CHRONIC AND INTERVENTIONAL PAIN BRIEF TECHNICAL REPORT The Erector Spinae Plane Block A Novel Analgesic Technique in Thoracic Neuropathic Pain Mauricio Forero, MD, FIPP,*Sanjib D. Adhikary, MD,† Hector Lopez, MD,‡ Calvin Tsui, BMSc,§ and Ki Jinn Chin, MBBS (Hons), MMed, FRCPC|| Case 1 Abstract: Thoracic neuropathic pain is a debilitating condition that is often poorly responsive to oral and topical pharmacotherapy. The benefit A 67-year-old man, weight 116 kg and height 188 cm [body of interventional nerve block procedures is unclear due to a paucity of ev- mass index (BMI), 32.8 kg/m2] with a history of heavy smoking idence and the invasiveness of the described techniques. In this report, we and paroxysmal supraventricular tachycardia controlled on ateno- describe a novel interfascial plane block, the erector spinae plane (ESP) lol, was referred to the chronic pain clinic with a 4-month history block, and its successful application in 2 cases of severe neuropathic pain of severe left-sided chest pain. A magnetic resonance imaging (the first resulting from metastatic disease of the ribs, and the second from scan of his thorax at initial presentation had been reported as nor- malunion of multiple rib fractures). In both cases, the ESP block also pro- mal, and the working diagnosis at the time of referral was post- duced an extensive multidermatomal sensory block. Anatomical and radio- herpetic neuralgia. He reported constant burning and stabbing logical investigation in fresh cadavers indicates that its likely site of action neuropathic pain of 10/10 severity on the numerical rating score is at the dorsal and ventral rami of the thoracic spinal nerves. -

Skeletal System? Skeletal System Chapters 6 & 7 Skeletal System = Bones, Joints, Cartilages, Ligaments
Warm-Up Activity • Fill in the names of the bones in the skeleton diagram. Warm-Up 1. What are the 4 types of bones? Give an example of each. 2. Give 3 ways you can tell a female skeleton from a male skeleton. 3. What hormones are involved in the skeletal system? Skeletal System Chapters 6 & 7 Skeletal System = bones, joints, cartilages, ligaments • Axial skeleton: long axis (skull, vertebral column, rib cage) • Appendicular skeleton: limbs and girdles Appendicular Axial Skeleton Skeleton • Cranium (skull) • Clavicle (collarbone) • Mandible (jaw) • Scapula (shoulder blade) • Vertebral column (spine) • Coxal (pelvic girdle) ▫ Cervical vertebrae • Humerus (arm) ▫ Thoracic vertebrae • Radius, ulna (forearm) ▫ Lumbar vertebrae • Carpals (wrist) • Metacarpals (hand) ▫ Sacrum • Phalanges (fingers, toes) ▫ Coccyx • Femur (thigh) • Sternum (breastbone) • Tibia, fibula (leg) • Ribs • Tarsal, metatarsals (foot) • Calcaneus (heel) • Patella (knee) Functions of the Bones • Support body and cradle soft organs • Protect vital organs • Movement: muscles move bones • Storage of minerals (calcium, phosphorus) & growth factors • Blood cell formation in bone marrow • Triglyceride (fat) storage Classification of Bones 1. Long bones ▫ Longer than they are wide (eg. femur, metacarpels) 2. Short bones ▫ Cube-shaped bones (eg. wrist and ankle) ▫ Sesamoid bones (within tendons – eg. patella) 3. Flat bones ▫ Thin, flat, slightly curved (eg. sternum, skull) 4. Irregular bones ▫ Complicated shapes (eg. vertebrae, hips) Figure 6.2 • Adult = 206 bones • Types of bone -

Pain Pattern Explanation Forms
Pain Pattern Explanation Forms 1. Cervical Facet Pain Pattern 2. Cervical Radicular/Dynatome Pain Pattern 3. Costotransverse Joint Pain Pattern 4. Fibromyalgia Points 5. Hip Joint Pain Pattern 6. Lumbar Dermatomes: Chemical Radiculitis 7. Lumbar Dermatomes: Disc Pathology 8. Lumbar Disc Pathology Healed 9. Lumbar Epidural Fibrosis 10. Lumbar Facet Pain Pattern 11. Lumbar Stenosis 12. Sacroiliac Joint Pain Pattern 13. Thoracic Facet Pain Pattern 14. Upper Cervical Joint Pain Pattern The OEA pain pattern handouts are PDF files that can be used for patient education and marketing. They help you explain your diagnosis with original illustrations that the patients can take home with them. There is limited text so you can tell your explanation of the treatment plan. The pain patterns can be printed in color or black and white. Once purchased, our business card will be replaced with yours to personalize each handout. Each illustration is based on the pain patterns that have been established in books or research articles when available. Normal anatomy and pathoanatomy illustrations are shown for the clinician to explain the diagnosis to the patient and how their treatment can influence the pain generator. These can also be utilized as marketing tools. The following pages are some guidelines that can be utilized to explain the handouts to patients. Cervical Facet Pain Pattern The cervical facet joints are the joints of the neck. Neurophysiologic studies have shown that cervical facet‐joint capsules are sources of neck pain.1 Dwyer et al.2 established pain patterns of the cervical facet joints. o Parasagittal cervical and cervicothoracic pain. -
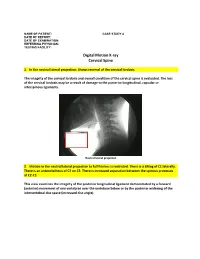
Digital Motion X-Ray Cervical Spine
NAME OF PATIENT: CASE STUDY 4 DATE OF REPORT: DATE OF EXAMINATION: REFERRING PHYSICIAN: TESTING FACILITY: Digital Motion X-ray Cervical Spine 1. In the neutral lateral projection: Shows reversal of the cervical lordosis. The integrity of the cervical lordosis and overall condition of the cervical spine is evaluated. The loss of the cervical lordosis may be a result of damage to the posterior longitudinal, capsular or interspinous ligaments. Neutral lateral projection 2. Motion in the neutral lateral projection to full flexion: Is restricted. There is a tilting of C1 laterally. There is an anterolisthesis of C2 on C3. There is increased separation between the spinous processes at C2-C3. This view examines the integrity of the posterior longitudinal ligament demonstrated by a forward (anterior) movement of one vertebrae over the vertebrae below or by the posterior widening of the intervertebral disc space (increased disc angle). Widening of posterior disc space Anterolisthesis The integrity of the interspinous ligament is evaluated in the lateral flexion view. Damage to this ligament results in increased separation of the spinous processes in flexion. Damaged Interspinous Ligament Full flexion projection 3. Motion in the neutral lateral projection to full extension: Is restricted. There is a retrolisthesis of C4 on C5. This view examines the integrity of the anterior longitudinal ligament demonstrated by a backward (posterior) movement of one vertebrae over the vertebrae below or by the anterior widening of the intervertebral disc space (increased disc angle). Retrolisthesis Widening of the anterior disc Full Extension 4. Motion in the oblique flexion projection: Is restricted. There is gapping of the facet joints at C6-C7 bilaterally and C7-T1 bilaterally. -

Anatomical Study of the Superior Cluneal Nerve and Its Estimation of Prevalence As a Cause of Lower Back Pain in a South African Population
Anatomical study of the superior cluneal nerve and its estimation of prevalence as a cause of lower back pain in a South African population by Leigh-Anne Loubser (10150804) Dissertation to be submitted in full fulfilment of the requirements for the degree Master of Science in Anatomy In the Faculty of Health Science University of Pretoria Supervisor: Prof AN Van Schoor1 Co-supervisor: Dr RP Raath2 1 Department of Anatomy, University of Pretoria 2 Netcare Jakaranda Hospital, Pretoria 2017 DECLARATION OF ORIGINALITY UNIVERSITY OF PRETORIA The Department of Anatomy places great emphasis upon integrity and ethical conduct in the preparation of all written work submitted for academic evaluation. While academic staff teach you about referencing techniques and how to avoid plagiarism, you too have a responsibility in this regard. If you are at any stage uncertain as to what is required, you should speak to your lecturer before any written work is submitted. You are guilty of plagiarism if you copy something from another author’s work (e.g. a book, an article, or a website) without acknowledging the source and pass it off as your own. In effect, you are stealing something that belongs to someone else. This is not only the case when you copy work word-for-word (verbatim), but also when you submit someone else’s work in a slightly altered form (paraphrase) or use a line of argument without acknowledging it. You are not allowed to use work previously produced by another student. You are also not allowed to let anybody copy your work with the intention of passing if off as his/her work. -

Relationship Between Endplate Defects, Modic Change, Facet Joint Degeneration, and Disc Degeneration of Cervical Spine
Neurospine 2020;17(2):443-452. Neurospine https://doi.org/10.14245/ns.2040076.038 pISSN 2586-6583 eISSN 2586-6591 Original Article Relationship Between Endplate Corresponding Author Defects, Modic Change, Facet Joint Dong Wuk Son https://orcid.org/0000-0002-9154-1923 Degeneration, and Disc Degeneration Department of Neurosurgery, Pusan of Cervical Spine National University Yangsan Hospital, 20 Geumo-ro, Mulgeum-eup, Yangsan 50612, Su-Hun Lee1,2, Dong Wuk Son1,2, Jun-Seok Lee1,2, Soon-Ki Sung1,2, Sang Weon Lee1,2, Korea Geun Sung Song1,2 E-mail: [email protected] 1Department of Neurosurgery, Pusan National University Yangsan Hospital, Pusan National University School of Medicine, Yangsan, Korea Received: February 11, 2020 2Research Institute for Convergence of Biomedical Science and Technology, Pusan National University Yangsan Revised: February 26, 2020 Hospital, Yangsan, Korea Accepted: February 27, 2020 Objective: The ‘‘disc degeneration precedes facet joint osteoarthritis’’ hypothesis and multi- dimensional analysis were actively discussed in lumbar spine. However, in cervical spine degeneration, the multifactorial analyzes of disc degeneration (DD), Modic changes (Mcs), facet degeneration, and endplate degeneration (ED) is still limited. In this cross-sectional study, we aimed to analyze the prevalence and interrelationship of cervical DD parameters. Methods: We retrospectively recruited 62 patients aged between 60 and 70 years. The disc height, segmental angle, ossified posterior longitudinal ligament (OPLL), ED, facet joint degeneration -

Ossification of the Posterior Longitudinal Ligament: Pathogenesis, Management, and Current Surgical Approaches
Neurosurg Focus 30 (3):E10, 2011 Ossification of the posterior longitudinal ligament: pathogenesis, management, and current surgical approaches A review ZACHARY A. SMITH, M.D.,1 COLIN C. BUCHANAN, M.D.,2 DAN RAPHAEL, P.A.-C.,1 AND LARRY T. KHOO, M.D.1 1Division of Neurosurgery, The Spine Clinic of Los Angeles, Good Samaritan Hospital, An Affiliate Hospital of the University of Southern California Medical School; and 2Department of Neurosurgery, Ronald Reagan–UCLA Medical Center, David Geffen School of Medicine at UCLA, Los Angeles, California Ossification of the posterior longitudinal ligament (OPLL) is an important cause of cervical myelopathy that results from bony ossification of the cervical or thoracic posterior longitudinal ligament (PLL). It has been estimated that nearly 25% of patients with cervical myelopathy will have features of OPLL. Patients commonly present in their mid-40s or 50s with clinical evidence of myelopathy. On MR and CT imaging, this can be seen as areas of ossification that commonly coalesce behind the cervical vertebral bodies, leading to direct ventral compression of the cord. While MR imaging will commonly demonstrate associated changes in the soft tissue, CT scanning will better define areas of ossification. This can also provide the clinician with evidence of possible dural ossification. The surgical management of OPLL remains a challenge to spine surgeons. Surgical alternatives include anterior, posterior, or circumferential decompression and/or stabilization. Anterior cervical stabilization options include cervical corpectomy or multilevel anterior cervical corpectomy and fusion, while posterior stabilization approaches include instrumented or noninstru- mented fusion or laminoplasty. Each of these approaches has distinct advantages and disadvantages. -

Ganglion Cyst of the Posterior Longitudinal Ligament Causing Lumbar Radiculopathy: Case Report
Spinal Cord (1997) 35, 632 ± 635 1997 International Medical Society of Paraplegia All rights reserved 1362 ± 4393/97 $12.00 Ganglion cyst of the posterior longitudinal ligament causing lumbar radiculopathy: case report Hisatoshi Baba1, Nobuaki Furusawa1, Yasuhisa Maezawa1, Kenzo Uchida1, Yasuo Kokubo1, Shinichi Imura1 and Sakon Noriki2 1Department of Orthopaedic Surgery; 2The First Department of Pathology, Fukui Medical School, Shimoaizuki 23, Matsuoka, Fukui 910-11, Japan We describe a man aged 26 years who presented with a neurological syndrome, which was found on lumbar radioculopathy to be due to a ganglion cyst originating from the posterior longitudinal ligament. Based on MRI ®ndings, a cystic lesion was suspected, a round lesion at L4 level with no connection to the adjacent facet or to the dura matter. During surgery, a liquid-containing cystic lesion was found to originate from the posterior longitudinal ligament at L4 level. The resected cyst was diagnosed histologically as a ganglion cyst. A complete cure was established after surgery and no recurrence was noted at a follow-up 1.7 years postoperatively. A ganglion cyst of the posterior longitudinal ligament should be considered in the dierential diagnosis of a cyst in the lumbar region causing neurological complications. Keywords: lumbar spine; posterior longitudinal ligament; ganglion cyst; radiculopathy Introduction Cystic lesions within the lumbosacral spinal canal can L4 dermatome. Muscle strength of the quadriceps cause symptoms and signs such as radiculopathy. femoris, tibialis anterior, extensor hallucis longus, was These cysts include ganglion1±3 and synovial cysts.4±7 assessed as normal. The straight leg raising test was To our knowledge, no report has previously been positive on the right at 508 and the femoral nerve described of a ganglion cyst in the posterior long- stretch test was also positive. -

Canine Thoracic Costovertebral and Costotransverse Joints Three Case Reports of Dysfunction and Manual Therapy Guidelines for A
Topics in Compan An Med 29 (2014) 1–5 Topical review Canine Thoracic Costovertebral and Costotransverse Joints: Three Case Reports of Dysfunction and Manual Therapy Guidelines for Assessment and Treatment of These Structures Laurie Edge-Hughes, BScPT, MAnimSt (Animal Physiotherapy), CAFCI, CCRTn Keywords: The costovertebral and costotransverse joints receive little attention in research. However, pain costovertebral associated with rib articulation dysfunction is reported to occur in human patients. The anatomic costotransverse structures of the canine rib joints and thoracic spine are similar to those of humans. As such, it is ribs physical therapy proposed that extrapolation from human physical therapy practice could be used for the assessment and rehabilitation treatment of the canine patient with presumed rib joint pain. This article presents 3 case studies that manual therapy demonstrate signs of rib dysfunction and successful treatment using primarily physical therapy manual techniques. General assessment and select treatment techniques are described. & 2014 Elsevier Inc. All rights reserved. The Canine Fitness Centre Ltd, Calgary, Alberta, Canada nAddress reprint requests to Laurie Edge-Hughes, BScPT, MAnimSt (Animal Physiotherapy), CAFCI, CCRT, The Canine Fitness Centre Ltd, 509—42nd Ave SE, Calgary, Alberta, Canada T2G 1Y7 E-mail: [email protected] The articular structures of the thorax comprise facet joints, the erect spine and further presented that in reviewing the literature, intervertebral disc, and costal joints. Little research has been they were unable to find mention of natural development of conducted on these joints in human or animal medicine. However, idiopathic scoliosis in quadrupeds; however, there are reports of clinical case presentations in human journals, manual therapy avian models and adolescent models in man. -

Posterior Longitudinal Ligament Status in Cervical Spine Bilateral Facet Dislocations
Thomas Jefferson University Jefferson Digital Commons Department of Orthopaedic Surgery Faculty Papers Department of Orthopaedic Surgery November 2005 Posterior longitudinal ligament status in cervical spine bilateral facet dislocations John A. Carrino Harvard Medical School & Brigham and Women's Hospital Geoffrey L. Manton Thomas Jefferson University Hospital William B. Morrison Thomas Jefferson University Hospital Alex R. Vaccaro Thomas Jefferson University Hospital and The Rothman Institute Mark E. Schweitzer New York University & Hospital for Joint Diseases Follow this and additional works at: https://jdc.jefferson.edu/orthofp Part of the Orthopedics Commons LetSee next us page know for additional how authors access to this document benefits ouy Recommended Citation Carrino, John A.; Manton, Geoffrey L.; Morrison, William B.; Vaccaro, Alex R.; Schweitzer, Mark E.; and Flanders, Adam E., "Posterior longitudinal ligament status in cervical spine bilateral facet dislocations" (2005). Department of Orthopaedic Surgery Faculty Papers. Paper 3. https://jdc.jefferson.edu/orthofp/3 This Article is brought to you for free and open access by the Jefferson Digital Commons. The Jefferson Digital Commons is a service of Thomas Jefferson University's Center for Teaching and Learning (CTL). The Commons is a showcase for Jefferson books and journals, peer-reviewed scholarly publications, unique historical collections from the University archives, and teaching tools. The Jefferson Digital Commons allows researchers and interested readers anywhere in the world to learn about and keep up to date with Jefferson scholarship. This article has been accepted for inclusion in Department of Orthopaedic Surgery Faculty Papers by an authorized administrator of the Jefferson Digital Commons. For more information, please contact: [email protected].