Lingual Lymph Node Metastases As a Prognostic Factor in Oral Squamous Cell Carcinoma—A Retrospective Multicenter Study
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Gross Anatomy of the Head and Neck Date: 26Th April 2020
MATRIC NO.: 17/MHS01/302 ASSIGNMENT TITTLE: NOSE AND ORAL CAVITY COURSE TITTLE: GROSS ANATOMY OF THE HEAD AND NECK DATE: 26TH APRIL 2020 QUESTION 1 Discuss the anatomy of the tongue, and comment on its applied anatomy ANSWER TONGUE: The tongue is a mobile muscular organ covered with mucous membrane. It can assume a variety of shapes and positions. It is partly in the oral cavity and partly in the oropharynx. The tongue’s main functions are articulation (forming words during speaking) and squeezing food into the oropharynx as part of deglutition (swallowing). The tongue is also involved with mastication, taste, and oral cleansing. It has importance in the digestive system and is the primary organ of taste in the gustatory system. The human tongue is divided into two parts; an oral part at the front and a pharyngeal part at the back. The left and right sides of the tongue are separated by a fibrous tissue called the lingual septum that results in a groove, the median sulcus on the tongue’s surface. PARTS OF THE TONGUE The tongue has a root, body, and apex. The root of the tongue is the attached posterior portion, extending between the mandible, hyoid, and the nearly vertical posterior surface of the tongue. The body of the tongue is the anterior, approximately two thirds of the tongue between root and apex. The apex (tip) of the tongue is the anterior end of the body, which rests against the incisor teeth. The body and apex of the tongue are extremely mobile. A midline groove divides the anterior part of the tongue into right and left parts. -

List of Questions for Students of Clinical Orofacial Anatomy (B01158)
List of questions for students of clinical orofacial anatomy (B01158) 1st branch of the trigeminal nerve (V1), ciliary ganglion + pupillar reflex 2nd branch of the trigeminal nerve (V2), pterygopalatine ganglion 3rd branch of the trigeminal nerve (V3) Anaesthesia of the upper jaw (intra- and extraoral) – anatomical background Applied anatomy of the hard and soft palati: lines A, H (Hauptmayer); palatal indexes, resiliency Buccal region Carotid triangle Cervical spaces and their connections in relation to the spreading of pathological processes Cervical sympathetic system CN IX, X, XI (only cervical part; ganglions, nuclei) CN XII, cervical ansae, cervical plexus CN.VII. ( branches, palsy types) Compression of the arteries: external carotid, facial, lingual, and superficial temporal Coniotomy. Tracheotomy. Anatomical background Determination of the occlusal plane in toothed and toothless jaws. Anatomical aspects. Camper plane. Developmental mechanism of the soft and hard palate, clefts Eruption of the permanent and deciduous teeth External carotid artery (course, branches, topographic relations) Extraglossal tongue muscles Face lines, face profile, facial indexes Face thirds. Middle third; fractures of the facial bones Forms of the dental arches and jaw forms during development Gingivodental region Hard palate (mucous membrane zones, development) Head parasympathetics (nuclei, ganglions, target organs) Inflammation spreading from teeth tops in the lower jaw Inflammation spreading from the region of the lower third molar Inflammation spreading -

Functional Anatomy of the Digestive System»
«Functional anatomy of the digestive system» KNMU, Department of human anatomy, Associate professor, PhD, Lupyr Marina Theme: The functional anatomy of the digestive system. Plan 1. The processes of digestion. 2. The basic functions of the compartments of the digestive system. 3. The review of a structure of the digestive system - the oral region - the pharynx - the esophagus - the stomach - the small intestine - the large intestine -the liver - the pancreas - peritoneum The Digestive System (systema digestorium) is a complex of organs whose function consists in mechanical and chemical treatment of the food, absorption of the treated nutrients and excretion of undigested remnants of the food. The processes of digestion consist of: 1. ingestion, or eating; 2. peristalsis, or involuntary sequential muscular contractions that move ingested nutriens along the digestive tract; 3. digestion, or the conversion of large nutrient particles into small molecules; 4. absorption, or the passage of usable nutrient molecules from the small intestine into the blood stream and lymphatic system. 5. defecation, or the elimination from the body of undigested and unabsorbed material as a solid waste. Cavity of the mouth • The digestive system has following functions: • In the mouth the gustatory sence, the temperature and the consistence of the food are determined. The teeth chew food and soliva from the solivary glands is added to the food to facilitate the formation of the manageable bolus. • In the saliva there is the proteino-mucous substance (mucin) and protein (lisocim). Mucin washes the food and breaks up the storch a little. And lisocim renders some hormfull substances. Usually food is in the cavity of the mouth during 15-16 sec. -

Adaptations of the Cetacean Hyolingual Apparatus for Aquatic Feeding and Thermoregulation
THE ANATOMICAL RECORD 290:546–568 (2007) Adaptations of the Cetacean Hyolingual Apparatus for Aquatic Feeding and Thermoregulation ALEXANDER J. WERTH* Department of Biology, Hampden-Sydney College, Hampden-Sydney, Virginia ABSTRACT Foraging methods vary considerably among semiaquatic and fully aquatic mammals. Semiaquatic animals often find food in water yet con- sume it on land, but as truly obligate aquatic mammals, cetaceans (whales, dolphins, and porpoises) must acquire and ingest food under- water. It is hypothesized that differences in foraging methods are reflected in cetacean hyolingual apparatus anatomy. This study compares the musculoskeletal anatomy of the hyolingual apparatus in 91 cetacean specimens, including 8 mysticetes (baleen whales) in two species and 91 odontocetes (toothed whales) in 11 species. Results reveal specific adapta- tions for aquatic life. Intrinsic fibers are sparser and extrinsic muscula- ture comprises a significantly greater proportion of the cetacean tongue relative to terrestrial mammals and other aquatic mammals such as pin- nipeds and sirenians. Relative sizes and connections of cetacean tongue muscles to the hyoid apparatus relate to differences in feeding methods used by cetaceans, specifically filtering, suction, and raptorial prehension. In odontocetes and eschrichtiids (gray whales), increased tongue muscula- ture and enlarged hyoids allow grasping and/or lingual depression to gen- erate intraoral suction for prey ingestion. In balaenopterids (rorqual whales), loose and flaccid tongues enable great distention of the oral cav- ity for prey engulfing. In balaenids (right and bowhead whales), large but stiffer tongues direct intraoral water flow for continuous filtration feed- ing. Balaenid and eschrichtiid (and possibly balaenopterid) mysticete tongues possess vascular retial adaptations for thermoregulation and large amounts of submucosal adipose tissue for nutritional storage. -

SPLANCHNOLOGY Part I. Digestive System (Пищеварительная Система)
КАЗАНСКИЙ ФЕДЕРАЛЬНЫЙ УНИВЕРСИТЕТ ИНСТИТУТ ФУНДАМЕНТАЛЬНОЙ МЕДИЦИНЫ И БИОЛОГИИ Кафедра морфологии и общей патологии А.А. Гумерова, С.Р. Абдулхаков, А.П. Киясов, Д.И. Андреева SPLANCHNOLOGY Part I. Digestive system (Пищеварительная система) Учебно-методическое пособие на английском языке Казань – 2015 УДК 611.71 ББК 28.706 Принято на заседании кафедры морфологии и общей патологии Протокол № 9 от 18 апреля 2015 года Рецензенты: кандидат медицинских наук, доцент каф. топографической анатомии и оперативной хирургии КГМУ С.А. Обыдённов; кандидат медицинских наук, доцент каф. топографической анатомии и оперативной хирургии КГМУ Ф.Г. Биккинеев Гумерова А.А., Абдулхаков С.Р., Киясов А.П., Андреева Д.И. SPLANCHNOLOGY. Part I. Digestive system / А.А. Гумерова, С.Р. Абдулхаков, А.П. Киясов, Д.И. Андреева. – Казань: Казан. ун-т, 2015. – 53 с. Учебно-методическое пособие адресовано студентам первого курса медицинских специальностей, проходящим обучение на английском языке, для самостоятельного изучения нормальной анатомии человека. Пособие посвящено Спланхнологии (науке о внутренних органах). В данной первой части пособия рассматривается анатомическое строение и функции системы в целом и отдельных органов, таких как полость рта, пищевод, желудок, тонкий и толстый кишечник, железы пищеварительной системы, а также расположение органов в брюшной полости и их взаимоотношения с брюшиной. Учебно-методическое пособие содержит в себе необходимые термины и объём информации, достаточный для сдачи модуля по данному разделу. © Гумерова А.А., Абдулхаков С.Р., Киясов А.П., Андреева Д.И., 2015 © Казанский университет, 2015 2 THE ALIMENTARY SYSTEM (systema alimentarium/digestorium) The alimentary system is a complex of organs with the function of mechanical and chemical treatment of food, absorption of the treated nutrients, and excretion of undigested remnants. -

6 Neoplasms of the Oral Cavity
Neoplasms of the Oral Cavity 103 6 Neoplasms of the Oral Cavity Marc Keberle CONTENTS regions laterally, the circumvallate papillae and the anterior tonsillar pillar dorsally, and the hard palate 103 6.1 Anatomy cranially. The center of the oral cavity is fi lled out by 6.1.1 The Floor of the Mouth 103 6.1.2 The Tongue 103 the tongue. 6.1.3 The Lips and Gingivobuccal Regions 104 6.1.4 The Hard Palate and the Region of the Retromolar Trigone 105 6.1.1 107 6.1.5 Lymphatic Drainage The Floor of the Mouth 6.2 Preferred Imaging Modalities 107 6.3 Pathology 107 6.3.1 Benign Lesions 107 The fl oor of the mouth is considered the space be- 6.3.1.1 Congenital Lesions 107 tween the mylohyoid muscle and the caudal mucosa 6.3.1.2 Infl ammatory Conditions 111 of the oral cavity. The mylohyoid muscle has the form 112 6.3.1.3 Benign Tumors of a hammock which is attached to the mandible ven- 6.3.2 Squamous Cell Cancer 114 6.3.2.1 General Considerations 114 trally and laterally on both sides but with a free dorsal 6.3.2.2 Lip Cancer 117 margin. Coronal planes nicely demonstrate the anat- 6.3.2.3 Floor of the Mouth Cancer 117 omy of the mylohyoid as well as the geniohyoid mus- 6.3.2.4 Retromolar Trigone Cancer 118 cles (Figs. 6.4, 6.5). The geniohyoid muscles are paired 119 6.3.2.5 Tongue Cancer sagittally orientated slender muscles on the superior 6.3.2.6 Hard Palate, Gingival and Buccal Cancer 120 6.3.3 Other Malignant Tumors 122 surface of the mylohyoid muscle. -

General Anatomy of Gastro-Intestinal System
General Anatomy of Gastro-IntesTinal System The teeth, Oral cavity, Tongue, Salivary glands, Pharynx. Their vessels and innervation IKIvo Klepáček Primordium of the alimentary canal (GastroInTestinal Canal) GIT devel– systema gastropulmonale – it develops from the embryonal intestine (entoderm) ; lower respiratory structurses are splitted from intewstine as a tracheobronchial pouch Ventral (head) intestine part is added to ectodermal pouch called stomodeum, caudal part of the intestine is added to ectodermal pouch called proctodeum Division of the alimentary tract: 1) oral ectodermal segment 2) main entodermal segment 3) caudal ectodermal segment děivision of the main segment: ventral gut (foregut – to biliary duct opening) middle gut (midgut – to 2/3 colon) IKdorsal gut (hindgut – to upper part of the anal canal Digestive System: Oral cavity (ectodermal origin) The gut and ist derivatives (entodermal origin) is devided in four sections: 1. Pharyngeal gut or pharynx 2. Foregut - esophagus, stomach, ¼ of duodenum, liver and gallblader, pancreas 3. Midgut – ¾ of duodenum, jejujnum, ilium, colon caecum, colon ascendens and 2/3 of colon transversum 4. Hindgut – 1/3 of colon transversum, colon descendens, colon sigmoideum, colon rectum, IKcanalis analis IK Alimentary tube (canal) - general structure – tunica mucosa (mucous membrane 1 • epithelium • lamina propria mucosae (lymph tissue) • lamina muscularis mucosae – tunica submucosa (submucous layer) – vessels, erves (plexus submucosus Meissneri) – tunica muscularis externa 7 (outer -

Palate, Tonsil, Pharyngeal Wall & Mouth and Tongue
Mouth and Tongue 口腔 與 舌頭 解剖學科 馮琮涵 副教授 分機 3250 E-mail: [email protected] Outline: • Skeletal framework of oral cavity • The floor (muscles) of oral cavity • The structure and muscles of tongue • The blood vessels and nerves of tongue • Position, openings and nerve innervation of salivary glands • The structure of soft and hard palates Skeletal framework of oral cavity • Maxilla • Palatine bone • Sphenoid bone • Temporal bone • Mandible • Hyoid bone Oral Region Oral cavity – oral vestibule and oral cavity proper The lips – covered by skin, orbicularis muscle & mucous membrane four parts: cutaneous zone, vermilion border, transitional zone and mucosal zone blood supply: sup. & inf. labial arteries – branches of facial artery sensory nerves: infraorbital nerve (CN V2) and mental nerve (CN V3) lymph: submandibular and submental lymph nodes The cheeks – the same structure as the lips buccal fatpad, buccinator muscle, buccal glands parotid duct – opening opposite the crown of the 2nd maxillary molar tooth The gingivae (gums) – fibrous tissue covered with mucous membrane alveolar mucosa (loose gingiva) & gingiva proper (attached gingiva) The floor of oral cavity • Mylohyoid muscle Nerve: nerve to mylohyoid (branch of inferior alveolar nerve) from mandibular nerve (CN V3) • Geniohyoid muscle Nerve: hypoglossal nerve (nerve fiber from cervical nerve; C1) The Tongue (highly mobile muscular organ) Gross features of the tongue Sulcus terminalis – foramen cecum Oral part (anterior 2/3) Pharyngeal part (posterior 1/3) Lingual frenulum, Sublingual caruncle -

(OC) and Its Accessory Organs Namely; the Tongue, Teeth, Salivary Glands
ORAL CAVITY The oral cavity (O.C) and its accessory organs namely; the tongue, teeth, salivary glands are concerned with the prehension, mastication and in salivation of food i.e. they are involved in the conversion of food for palatability. The O.C. extends from the lips into the entrance of the pharynx. The osseous support of the mouth is provided by premaxilla, palatine, alveoli processes of the maxilla, the horizontal part of the palatine bone dorsally, the mandibular rami laterally of the body of the mandible ventrally. The soft structures complying with the wall of the mouth are the cheeks laterally, the lips rostrally and the mylohyoid ventrally. Its dorsal limit or roof is the hard palate. Caudally, the oral cavity communicates with the oropharynx by a narrow opening called the Isthmus faucium formed by roof of the tongue and the soft palate, it is usually closed. When the jaws are closed, the mouth is divided by the teeth and the alveolar processes into vestibuble and oral cavity proper. These two cavities communicates via the interdental spaces. The part of the vestibule between the incisors and the lips is the labial vestibule, while that between the check teeth and the check is the buccal Vestibule. Rostrally, two narrow incisive ducts connect the oral cavity and the nasal cavity. The duct opens on the incisive papillae. The mucus membrane of the O.C. is usually pink but may be pigmented (black) in some places. It is well supplied with blood vessels and in its sub mucosa it contains serous or mucous gland know as the labial, buccal and lingual glands (depending on their location). -
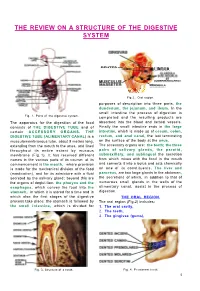
The Review on a Structure of the Digestive System
THE REVIEW ON A STRUCTURE OF THE DIGESTIVE SYSTEM Fig 2.. Oral region. purposes of description into three parts, the duodenum, the jejunum, and ileum. In the small intestine the process of digestion is Fig. 1. Parts of the digestive system. completed and the resulting products are The apparatus for the digestion of the food absorbed into the blood and lacteal vessels. consists of THE DIGESTIVE TUBE and of Finally the small intestine ends in the large certain ACCESSORY ORGANS. THE intestine, which is made up of cecum, colon, DIGESTIVE TUBE (ALIMENTARY CANAL) is a rectum, and anal canal, the last terminating musculomembranous tube, about 9 metres long, on the surface of the body at the anus. extending from the mouth to the anus, and lined The accessory organs are: the teeth; the three throughout its entire extent by mucous pairs of salivary glands, the parotid, membrane (Fig. 1). It has received different submaxillary, and sublingual the secretion names in the various parts of its course: at its from which mixes with the food in the mouth commencement is the mouth, where provision and converts it into a bolus and acts chemically is made for the mechanical division of the food on one of its constituents. The liver and (mastication), and for its admixture with a fluid pancreas, are two large glands in the abdomen, secreted by the salivary gland; beyond this are the secretions of which, in addition to that of the organs of deglutition, the pharynx and the numerous small glands in the walls of the esophagus, which convey the food into the alimentary canal, assist in the process of stomach, in which it is stored for a time and in digestion. -

Magnetic Resonance Imaging of the Normal Tongue: Qualitative Evaluation of Fat-Suppressed Contrast Enhanced Images
Bulletin of the Osaka Medical College 49 1, 2 21-28, 2003 21 Original Article Magnetic Resonance Imaging of the Normal Tongue: Qualitative Evaluation of Fat-suppressed Contrast Enhanced Images Yasunori ARIYOSHI1), Masashi SHIMAHARA1), Yasuo UESUGI2), Isamu NARABAYASHI2) 1) Department of Oral Surgery, Osaka Medical College 2) Department of Radiology, Osaka Medical College Key Words : magnetic resonance imaging, fat suppression, tongue, anatomy ABSTRACT Objective: For diagnosing the lesions on magnetic resonance imaging (MRI), it is necessary to understand normal structures on each sequence. In this study, we attempted to clarify structures of the normal tongue on fat -suppressed enhanced MRI qualitatively. Clinical material and method: Twenty-seven fat-suppressed enhanced MR images of normal tongue were studied, which were obtained using a T1 weighted spin echo pulse sequence (T1WI- SE) with the chemical shift selective (CHESS) method by a superconducting MRI scanner operating at 1.5T. Tongue structures and their signal intensities on fat-suppressed enhanced images were assessed and compared to those obtained by non-enhanced T1WI. Results: Normal tongues were found to be composed of a symmetrical high signal area (HSA), low signal area (LSA), lingual septum, bilateral sublingual gland, and genioglossus muscle on non- enhanced T1WI. In the fat-suppressed enhanced images, HSA and lingual septum signal intensities were suppressed. Further, though the lingual mucosa was well visualized in fat-suppressed enhanced images (P<0.05), differentiation of HSA and LSA was difficult as compared to the non- enhanced scans (P<0.05). Conclusion: Fat-suppressed enhanced scans demonstrated simple anatomical structures as com- pared to conventional T1WI with independent signal intensity. -

List of Questions for Students of Clinical Orofacial Anatomy (B01158) Follows Present Accreditation This Year; Focused on Repetition of the Morphologic Knowledges
List of questions for students of clinical orofacial anatomy (B01158) Follows present accreditation this year; focused on repetition of the morphologic knowledges 1. External carotid artery (course, branches, topographic relations) Cervical sympathetic system Growth of the skull basis – resorptive and growth active structures; nasozygomaxillar complex 2. Lymphatic nodes and vessels in the neck and head (overview, resection of the lymph nodes an block) Temporomandibular joint (movements, condyle positions inside joint) Spatium parotideum and retromandibular fossa 3. Veins of the face and neck Pterygomandibular space Regio gingivodentalis 4. Otic ganglion + Jacobson´s anastomosis Lymph outflow from the upper and lower jaws, periodontium, vestibulum oris and tongue Sphenoid and ethmoid paranasal sinuses 5. Head parasympathetics (nuclei, ganglions, target organs) Temporal region Mandible. Development, growth and remodelling during life (Meckel and neck growth cartilages) 6. Dura mater, sinus durae matris, emissaries, vv. ophthalmicae Inflammation spreading from the teeth of the upper jaw Skin innervation of the head and neck (overview) 7. Oral mucosa (arrangement and overview of its morphologic structures) Maxillary and frontal sinuses, ostiomeatal unit Retropharyngeal space (borders, content), its relation to the other spaces; infection spreading 8. Hard palate (mucous membrane zones, development) Vestibulum oris (borders, folds, varieties) Compression of the arteries: external carotid, facial, lingual, and superficial temporal 9. Muscles of facial expression, modiole Carotid triangle Anaesthesia of the upper jaw (intra- and extraoral) – anatomical background 10. Parotid gland, structure, topographic relations and its nerve supply Inflammation spreading from teeth of the lower jaw Nasopharynx (its form, mucous relief and lymph structures) 11. Isthmus faucium, oropharynx and hypopharynx Face lines, face profile, facial indexes Lymph drainage of the tongue, nasopharynx and tonsills – overview st 12.