Federal Register/Vol. 86, No. 73/Monday, April 19, 2021/Rules
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
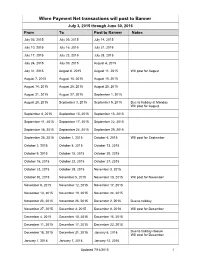
Transactions Posted to Pathway
When Payment Net transactions will post to Banner July 3, 2015 through June 30, 2016 From To Post to Banner Notes July 03, 2015 July 09, 2015 July 14, 2015 July 10, 2015 July 16, 2015 July 21, 2015 July 17, 2015 July 23, 2015 July 28, 2015 July 24, 2015 July 30, 2015 August 4, 2015 July 31, 2015 August 6, 2015 August 11, 2015 Will post for August August 7, 2015 August 13, 2015 August 18, 2015 August 14, 2015 August 20, 2015 August 25, 2015 August 21, 2015 August 27, 2015 September 1, 2015 August 28, 2015 September 3, 2015 September 9, 2015 Due to holiday at Monday Will post for August September 4, 2015 September 10, 2015 September 15, 2015 September 11, 2015 September 17, 2015 September 22, 2015 September 18, 2015 September 24, 2015 September 29, 2015 September 25, 2015 October 1, 2015 October 6, 2015 Will post for September October 2, 2015 October 8, 2015 October 13, 2015 October 9, 2015 October 15, 2015 October 20, 2015 October 16, 2015 October 22, 2015 October 27, 2015 October 23, 2015 October 29, 2015 November 3, 2015 October 30, 2015 November 5, 2015 November 10, 2015 Will post for November November 6, 2015 November 12, 2015 November 17, 2015 November 13, 2015 November 19, 2015 November 24, 2015 November 20, 2015 November 26, 2015 December 2, 2015 Due to holiday November 27, 2015 December 3, 2015 December 8, 2018 Will post for December December 4, 2015 December 10, 2015 December 15, 2015 December 11, 2015 December 17, 2015 December 22, 2015 December 18, 2015 December 31, 2015 January 6, 2016 Due to holiday closure Will post for December -

Early Dance Division Calendar 17-18
Early Dance Division 2017-2018 Session 1 September 9 – November 3 Monday Classes Tuesday Classes September 11 Class September 12 Class September 18 Class September 19 Class September 25 Class September 26 Class October 2 Class October 3 Class October 9 Class October 10 Class October 16 Class October 17 Class October 23 Class October 24 Class October 30 Last Class October 31 Last Class Wednesday Classes Thursday Classes September 13 Class September 14 Class September 20 Class September 21* Class September 27 Class September 28 Class October 4 Class October 5 Class October 11 Class October 12 Class October 18 Class October 19 Class October 25 Class October 26 Class November 1 Last Class November 2 Last Class Saturday Classes Sunday Classes September 9 Class September 10 Class September 16 Class September 17 Class September 23 Class September 24 Class September 30* Class October 1 Class October 7 Class October 8 Class October 14 Class October 15 Class October 21 Class October 22 Class October 28 Last Class October 29 Last Class *Absences due to the holiday will be granted an additional make-up class. Early Dance Division 2017-2018 Session 2 November 4 – January 22 Monday Classes Tuesday Classes November 6 Class November 7 Class November 13 Class November 14 Class November 20 No Class November 21 No Class November 27 Class November 28 Class December 4 Class December 5 Class December 11 Class December 12 Class December 18 Class December 19 Class December 25 No Class December 26 No Class January 1 No Class January 2 No Class January 8 Class -

2021-2022 Custom & Standard Information Due Dates
2021-2022 CUSTOM & STANDARD INFORMATION DUE DATES Desired Cover All Desired Cover All Delivery Date Info. Due Text Due Delivery Date Info. Due Text Due May 31 No Deliveries No Deliveries July 19 April 12 May 10 June 1 February 23 March 23 July 20 April 13 May 11 June 2 February 24 March 24 July 21 April 14 May 12 June 3 February 25 March 25 July 22 April 15 May 13 June 4 February 26 March 26 July 23 April 16 May 14 June 7 March 1 March 29 July 26 April 19 May 17 June 8 March 2 March 30 July 27 April 20 May 18 June 9 March 3 March 31 July 28 April 21 May 19 June 10 March 4 April 1 July 29 April 22 May 20 June 11 March 5 April 2 July 30 April 23 May 21 June 14 March 8 April 5 August 2 April 26 May 24 June 15 March 9 April 6 August 3 April 27 May 25 June 16 March 10 April 7 August 4 April 28 May 26 June 17 March 11 April 8 August 5 April 29 May 27 June 18 March 12 April 9 August 6 April 30 May 28 June 21 March 15 April 12 August 9 May 3 May 28 June 22 March 16 April 13 August 10 May 4 June 1 June 23 March 17 April 14 August 11 May 5 June 2 June 24 March 18 April 15 August 12 May 6 June 3 June 25 March 19 April 16 August 13 May 7 June 4 June 28 March 22 April 19 August 16 May 10 June 7 June 29 March 23 April 20 August 17 May 11 June 8 June 30 March 24 April 21 August 18 May 12 June 9 July 1 March 25 April 22 August 19 May 13 June 10 July 2 March 26 April 23 August 20 May 14 June 11 July 5 March 29 April 26 August 23 May 17 June 14 July 6 March 30 April 27 August 24 May 18 June 15 July 7 March 31 April 28 August 25 May 19 June 16 July 8 April 1 April 29 August 26 May 20 June 17 July 9 April 2 April 30 August 27 May 21 June 18 July 12 April 5 May 3 August 30 May 24 June 21 July 13 April 6 May 4 August 31 May 25 June 22 July 14 April 7 May 5 September 1 May 26 June 23 July 15 April 8 May 6 September 2 May 27 June 24 July 16 April 9 May 7 September 3 May 28 June 25. -

2021 7 Day Working Days Calendar
2021 7 Day Working Days Calendar The Working Day Calendar is used to compute the estimated completion date of a contract. To use the calendar, find the start date of the contract, add the working days to the number of the calendar date (a number from 1 to 1000), and subtract 1, find that calculated number in the calendar and that will be the completion date of the contract Date Number of the Calendar Date Friday, January 1, 2021 133 Saturday, January 2, 2021 134 Sunday, January 3, 2021 135 Monday, January 4, 2021 136 Tuesday, January 5, 2021 137 Wednesday, January 6, 2021 138 Thursday, January 7, 2021 139 Friday, January 8, 2021 140 Saturday, January 9, 2021 141 Sunday, January 10, 2021 142 Monday, January 11, 2021 143 Tuesday, January 12, 2021 144 Wednesday, January 13, 2021 145 Thursday, January 14, 2021 146 Friday, January 15, 2021 147 Saturday, January 16, 2021 148 Sunday, January 17, 2021 149 Monday, January 18, 2021 150 Tuesday, January 19, 2021 151 Wednesday, January 20, 2021 152 Thursday, January 21, 2021 153 Friday, January 22, 2021 154 Saturday, January 23, 2021 155 Sunday, January 24, 2021 156 Monday, January 25, 2021 157 Tuesday, January 26, 2021 158 Wednesday, January 27, 2021 159 Thursday, January 28, 2021 160 Friday, January 29, 2021 161 Saturday, January 30, 2021 162 Sunday, January 31, 2021 163 Monday, February 1, 2021 164 Tuesday, February 2, 2021 165 Wednesday, February 3, 2021 166 Thursday, February 4, 2021 167 Date Number of the Calendar Date Friday, February 5, 2021 168 Saturday, February 6, 2021 169 Sunday, February -

In Vitro Pharmacological Characterization of Buprenorphine
JPET Fast Forward. Published on August 14, 2018 as DOI: 10.1124/jpet.118.249839 This article has not been copyedited and formatted. The final version may differ from this version. JPET#249839 In Vitro Pharmacological Characterization of Buprenorphine, Samidorphan, and Combinations Being Developed as an Adjunctive Treatment for Major Depressive Disorder Downloaded from jpet.aspetjournals.org Jean M. Bidlack, Brian I. Knapp, Daniel R. Deaver, Margarita Plotnikava, Derrick Arnelle, Angela M. Wonsey, May Fern Toh, at ASPET Journals on September 28, 2021 Sokhom S. Pin, and Mark N. Namchuk Department of Pharmacology and Physiology, University of Rochester, School of Medicine and Dentistry, Rochester, NY 14642 (J.M.B., B.I.K.) and Alkermes, Inc., Waltham, MA 02451(D.R.D., M.P., D.A., A.M.W., M.F.T., S.S.P., M.N.N.) 1 JPET Fast Forward. Published on August 14, 2018 as DOI: 10.1124/jpet.118.249839 This article has not been copyedited and formatted. The final version may differ from this version. JPET#249839 Running Title: Buprenorphine and Samidorphan Pharmacological Properties Corresponding Author: Jean M. Bidlack, Ph.D., Department of Pharmacology and Physiology, P.O. Box 711, University of Rochester, School of Medicine and Dentistry, Rochester, New York 14642-8711, USA; Tel. (585) 275-5600; Fax (585) 273-2652; E-Mail: [email protected] Number of text pages: 24 Number of tables: 9 Number of figures: 7 and two supplementary figures Number of references: 42 Downloaded from Number of words in Abstract: 255 Number of words in Introduction: -

2020–2021District Calendar
2020–2021 DISTRICT CALENDAR August 10-14 ...........................New Leader Institute November 11 .................... Veterans Day: No school Major Religious & Cultural Holidays August 17-20 ........ BPS Learns Summer Conference November 25 ..... Early release for students and staff 2020 2021 (TSI, ALI, English Learner Symposium, November 26-27 ....Thanksgiving Recess: No school July 31 ..........Eid al-Adha Jan. 1 ........New Year’s Day Early Childhood/UPK Conference, December 24- January 1 ..Winter Recess: No school Sep. 19-20 ..Rosh Hashanah Jan. 6 ......Three Kings Day New Teacher Induction) January 4.................... All teachers and paras report . Sep. 28 .........Yom Kippur Feb. 12 .... Lunar New Year September 1 ......REMOTE: UP Academies: Boston, January 5...................... Students return from recess Nov. 14 ...... Diwali begins Feb. 17 ... Ash Wednesday Dorchester, and Holland, January 18..................M.L. King Jr. Day: No school all grades − first day of school Nov. 26 .......Thanksgiving Mar. 27-Apr. 3 ..... Passover February 15 .................... Presidents Day: No school September 7 .......................... Labor Day: No school Dec. 10-18 .......Hanukkah Apr. 2 ............ Good Friday February 16-19.............February Recess: No school September 8 .............. All teachers and paras report Dec. 25 ............Christmas Apr. 4 ...................... Easter April 2 ...................................................... No school September 21 ..........REMOTE: ALL Students report Dec. -
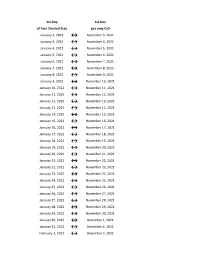
Flex Dates.Xlsx
1st Day 1st Day of Your Desired Stay you may Call January 2, 2022 ↔ November 3, 2021 January 3, 2022 ↔ November 4, 2021 January 4, 2022 ↔ November 5, 2021 January 5, 2022 ↔ November 6, 2021 January 6, 2022 ↔ November 7, 2021 January 7, 2022 ↔ November 8, 2021 January 8, 2022 ↔ November 9, 2021 January 9, 2022 ↔ November 10, 2021 January 10, 2022 ↔ November 11, 2021 January 11, 2022 ↔ November 12, 2021 January 12, 2022 ↔ November 13, 2021 January 13, 2022 ↔ November 14, 2021 January 14, 2022 ↔ November 15, 2021 January 15, 2022 ↔ November 16, 2021 January 16, 2022 ↔ November 17, 2021 January 17, 2022 ↔ November 18, 2021 January 18, 2022 ↔ November 19, 2021 January 19, 2022 ↔ November 20, 2021 January 20, 2022 ↔ November 21, 2021 January 21, 2022 ↔ November 22, 2021 January 22, 2022 ↔ November 23, 2021 January 23, 2022 ↔ November 24, 2021 January 24, 2022 ↔ November 25, 2021 January 25, 2022 ↔ November 26, 2021 January 26, 2022 ↔ November 27, 2021 January 27, 2022 ↔ November 28, 2021 January 28, 2022 ↔ November 29, 2021 January 29, 2022 ↔ November 30, 2021 January 30, 2022 ↔ December 1, 2021 January 31, 2022 ↔ December 2, 2021 February 1, 2022 ↔ December 3, 2021 1st Day 1st Day of Your Desired Stay you may Call February 2, 2022 ↔ December 4, 2021 February 3, 2022 ↔ December 5, 2021 February 4, 2022 ↔ December 6, 2021 February 5, 2022 ↔ December 7, 2021 February 6, 2022 ↔ December 8, 2021 February 7, 2022 ↔ December 9, 2021 February 8, 2022 ↔ December 10, 2021 February 9, 2022 ↔ December 11, 2021 February 10, 2022 ↔ December 12, 2021 February -

Removing Samidorphan from Schedule II
The Acting Administrator of the Drug Enforcement Administration issued an order to extend the temporary schedule I status of ethyl 2-(1-(5- fluoropentyl)-1H-indazole-3-carboxamido)-3,3-dimethylbutanoate (Other name: 5F-EDMB-PINACA); methyl 2-(1-(5-fluoropentyl)-1H-indole-3- carboxamido)-3,3-dimethylbutanoate (Other name: 5F-MDMB-PICA); N- (adamantan-1-yl)-1-(4-fluorobenzyl)-1H-indazole-3-carboxamide (Other names: FUB-AKB48, FUB-APINACA, AKB48, N-(4-fluorobenzyl)); 1-(5- fluoropentyl)-N-(2-phenylpropan-2-yl)-1H-indazole- 3-carboxamide (Others names: 5F-CUMYL-PINACA; SGT-25); and (1-(4- fluorobenzyl)-1H-indol-3-yl)(2,2,3,3-tetramethylcyclopropyl)methanone (Other name: FUB-144), and their optical, positional, and geometric isomers, salts, and salts of isomers. This order will extend the temporary scheduling of 5F-EDMB-PINACA, 5F-MDMB-PICA, FUB-AKB48, 5F-CUMYL- PINACA and FUB-144 for one year or until the permanent scheduling action for these substances is completed, whichever occurs first. This order was published in the March 31, 2021 issue of the Federal Register, Volume 86, Number 60, pages 16669-16670 and was effective April 16, 2021. The Acting Administrator of the Drug Enforcement Administration issued a final rule removing samidorphan (3-carboxamido-4-hydroxy naltrexone) and its salts from the schedules of the Controlled Substances Act. This final rule was published in the April 19, 2021 issue of the Federal Register, Volume 86, Number 73, pages 20284-20286 and was effective April 19, 2021. This action was based upon the following: 1. Samidorphan does not possess abuse or dependence potential, 2. -

2019-2022 Calendar of Major Jewish Holidays
2019-2022 CALENDAR OF MAJOR JEWISH HOLIDAYS Please note: Jewish students may not be able to participate in school activities that take place on the days marked with an *. 2019 2020 2021 2022 PURIM Celebrates the defeat of the plot to destroy March 21 March 10 February 26 March 17 the Jews of Persia. PASSOVER Deliverance of the Jewish people from Egypt. The first *Eve. of April 19 *Eve. of April 8 *Eve. of March 27 *Eve of April 15 and last two days are observed as full holidays. There are *April 20 *April 9 *March 28 *April 16 dietary restrictions against leavened products (such as *April 21 *April 10 *March 29 *April17 bread, pastries, pasta, certain legumes and more) during *April 26 *April 15 *April 3 *April 21 all eight days of the holiday. *April 27 *April 16 *April 4 *April 22 SHAVUOT *Eve. of June 8 *Eve. of May 28 *Eve. of May 16 *Eve of June 3 Feast of Weeks, marks the giving of the Law (Torah) *June 9 *May 29 *May 17 *June 4 at Mt. Sinai. (Often linked with the Confirmation *June 10 *May 30 *May 18 *June 5 of teenagers.) ROSH HASHANAH *Eve. of Sept. 29 *Eve. of Sept. 18 *Eve. of Sept. 6 *Eve of Sept 25 The Jewish New Year; start of the Ten Days of Penitence. *Sept. 30 *Sept. 19 *Sept. 7 *Sept. 26 The first two days are observed as full holidays. *Oct. 1 *Sept. 20 *Sept. 8 *Sept. 27 YOM KIPPUR Day of Atonement; the most solemn day *Eve. -

Flex Dates.Xlsx
1st Day 1st Day of Your Desired Stay you may Call January 3, 2021 ↔ November 4, 2020 January 4, 2021 ↔ November 5, 2020 January 5, 2021 ↔ November 6, 2020 January 6, 2021 ↔ November 7, 2020 January 7, 2021 ↔ November 8, 2020 January 8, 2021 ↔ November 9, 2020 January 9, 2021 ↔ November 10, 2020 January 10, 2021 ↔ November 11, 2020 January 11, 2021 ↔ November 12, 2020 January 12, 2021 ↔ November 13, 2020 January 13, 2021 ↔ November 14, 2020 January 14, 2021 ↔ November 15, 2020 January 15, 2021 ↔ November 16, 2020 January 16, 2021 ↔ November 17, 2020 January 17, 2021 ↔ November 18, 2020 January 18, 2021 ↔ November 19, 2020 January 19, 2021 ↔ November 20, 2020 January 20, 2021 ↔ November 21, 2020 January 21, 2021 ↔ November 22, 2020 January 22, 2021 ↔ November 23, 2020 January 23, 2021 ↔ November 24, 2020 January 24, 2021 ↔ November 25, 2020 January 25, 2021 ↔ November 26, 2020 January 26, 2021 ↔ November 27, 2020 January 27, 2021 ↔ November 28, 2020 January 28, 2021 ↔ November 29, 2020 January 29, 2021 ↔ November 30, 2020 January 30, 2021 ↔ December 1, 2020 January 31, 2021 ↔ December 2, 2020 February 1, 2021 ↔ December 3, 2020 February 2, 2021 ↔ December 4, 2020 1st Day 1st Day of Your Desired Stay you may Call February 3, 2021 ↔ December 5, 2020 February 4, 2021 ↔ December 6, 2020 February 5, 2021 ↔ December 7, 2020 February 6, 2021 ↔ December 8, 2020 February 7, 2021 ↔ December 9, 2020 February 8, 2021 ↔ December 10, 2020 February 9, 2021 ↔ December 11, 2020 February 10, 2021 ↔ December 12, 2020 February 11, 2021 ↔ December 13, 2020 -

August 25, 2021 NEW YORK FORWARD/REOPENING
September 24, 2021 NEW YORK FORWARD/REOPENING GUIDANCE & INFORMATIONi FEDERAL UPDATES: • On August 3, 2021, the Centers for Disease Control and Prevention (CDC) issued an extension of the nationwide residential eviction pause in areas experiencing substantial and high levels of community transmission levels of SARS-CoV-2, which is aligned with the mask order. The moratorium order, that expires on October 3, 2021, allows additional time for rent relief to reach renters and to further increase vaccination rates. See: Press Release ; Signed Order • On July 27, 2021, the Centers for Disease Control and Prevention (CDC) updated its guidance for mask wearing in public indoor settings for fully vaccinated people in areas where coronavirus transmission is high, in response to the spread of the Delta Variant. The CDC also included a recommendation for fully vaccinated people who have a known exposure to someone with suspected or confirmed COVID-19 to be tested 3-5 days after exposure, and to wear a mask in public indoor settings for 14 days or until they receive a negative test result. Further, the CDC recommends universal indoor masking for all teachers, staff, students, and visitors to schools, regardless of vaccination status See: https://www.cdc.gov/coronavirus/2019- ncov/vaccines/fully-vaccinated-guidance.html • The CDC on Thursday, June 24, 2021 announced a one-month extension to its nationwide pause on evictions that was executed in response to the pandemic. The moratorium that was scheduled to expire on June 30, 2021 is now extended through July 31, 2021 and this is intended to be the final extension of the moratorium. -

Patent Application Publication ( 10 ) Pub . No . : US 2019 / 0192440 A1
US 20190192440A1 (19 ) United States (12 ) Patent Application Publication ( 10) Pub . No. : US 2019 /0192440 A1 LI (43 ) Pub . Date : Jun . 27 , 2019 ( 54 ) ORAL DRUG DOSAGE FORM COMPRISING Publication Classification DRUG IN THE FORM OF NANOPARTICLES (51 ) Int . CI. A61K 9 / 20 (2006 .01 ) ( 71 ) Applicant: Triastek , Inc. , Nanjing ( CN ) A61K 9 /00 ( 2006 . 01) A61K 31/ 192 ( 2006 .01 ) (72 ) Inventor : Xiaoling LI , Dublin , CA (US ) A61K 9 / 24 ( 2006 .01 ) ( 52 ) U . S . CI. ( 21 ) Appl. No. : 16 /289 ,499 CPC . .. .. A61K 9 /2031 (2013 . 01 ) ; A61K 9 /0065 ( 22 ) Filed : Feb . 28 , 2019 (2013 .01 ) ; A61K 9 / 209 ( 2013 .01 ) ; A61K 9 /2027 ( 2013 .01 ) ; A61K 31/ 192 ( 2013. 01 ) ; Related U . S . Application Data A61K 9 /2072 ( 2013 .01 ) (63 ) Continuation of application No. 16 /028 ,305 , filed on Jul. 5 , 2018 , now Pat . No . 10 , 258 ,575 , which is a (57 ) ABSTRACT continuation of application No . 15 / 173 ,596 , filed on The present disclosure provides a stable solid pharmaceuti Jun . 3 , 2016 . cal dosage form for oral administration . The dosage form (60 ) Provisional application No . 62 /313 ,092 , filed on Mar. includes a substrate that forms at least one compartment and 24 , 2016 , provisional application No . 62 / 296 , 087 , a drug content loaded into the compartment. The dosage filed on Feb . 17 , 2016 , provisional application No . form is so designed that the active pharmaceutical ingredient 62 / 170, 645 , filed on Jun . 3 , 2015 . of the drug content is released in a controlled manner. Patent Application Publication Jun . 27 , 2019 Sheet 1 of 20 US 2019 /0192440 A1 FIG .