Anatomy and Physiology of Continence 1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Star Fish Early Development Amphioxus Gastrulation
Faculty of Biological Science and Technology Zoology and Botanical department Practical embryology Amphibian development (4 mm larvae; sagittal sections) By: Shirin Kashfi Ph.D in Animal Development [email protected] tail bud stage At the time of neurulation termination, a part which is known as tail bud develops in the end of embryo body The entire body elongate and shorten in dorso-ventral direction in some extend First muscular responses to external stimuli develop, so this stage refer as muscular response stage too Tail bud is elongated and contains neural tube, notochord and unsegmented mesoderm as well as dorsal and ventral tail fin Five primary brain vesicles are developed (telencephalon, diencephalon, mesencephalon, metencephalon and myelencephalon) Retina (neural retina and retinal pigmented epithelium) and lens placode are formed Auditory vesicle in associated with hind brain and olfactory placode in associated with forebrain (telencephalon) are developed Heart rudiment is developed below to pharynx from mesoderm Foregut, midgut and hindgut are formed. In foregut oral cavity are expanded ventrally and laterally, pharynx and liver diverticulum can be recognizable Pronephric kidney is developed from intermediate mesoderm Somites are formed stage 18, 96 hpf, 4 mm in all sections please consider anterior, posterior, dorsal and ventral directions surface ectoderm yolky endoderm yolky endoderm can be seen in these sections gradually head region is appeared From: embryo/amphibian development/Book - The Frog Its Reproduction -

2/2/2011 1 Development of Development of Endodermal
2/2/2011 ZOO 401- Embryology-Dr. Salah A. Martin DEVELOPMENT OF THE DIGESTIVE SYSTEM ◦ Primitive Gut Tube ◦ Proctodeum and Stomodeum ◦ Stomach Development of Endodermal Organs ◦ Duodenum ◦ Pancreas ◦ Liver and Biliary Apparatus ◦ Spleen ◦ Midgut Wednesday, February 02, 2011 DEVELOPMENT OF THE DIGESTIVE SYSTEM 2 Wednesday, February 02, 2011 Development of Ectodermal Organs 1 ZOO 401- Embryology-Dr. Salah A. Martin ZOO 401- Embryology-Dr. Salah A. Martin Primitive Gut Tube Proctodeum and Stomodeum The primitive gut tube is derived from the dorsal part of the yolk sac , which is incorporated into the body of The proctodeum (anal pit) is the primordial the embryo during folding of the embryo during the fourth week. anus , and the stomodeum is the primordial The primitive gut tube is divided into three sections. mouth . The epithelium of and the parenchyma of In both of these areas ectoderm is in direct glands associated with the digestive tract (e.g., liver and pancreas) are derived from endoderm . contact with endoderm without intervening The muscular walls of the digestive tract (lamina mesoderm, eventually leading to degeneration propria, muscularis mucosae, submucosa, muscularis of both tissue layers. Foregut, Esophagus. externa, adventitia and/or serosa) are derived from splanchnic mesoderm . The tracheoesophageal septum divides the During the solid stage of development the endoderm foregut into the esophagus and of the gut tube proliferates until the gut is a solid tube. trachea. information. A process of recanalization restores the lumen. Wednesday, February 02, 2011 Primitive Gut Tube 3 Wednesday, February 02, 2011 Proctodeum and Stomodeum 4 ZOO 401- Embryology-Dr. Salah A. -

Embryonic Development of the Chicken External Cloaca and Phallus
Scanning Electron Microscopy Volume 1986 Number 2 Article 35 5-23-1986 Embryonic Development of the Chicken External Cloaca and Phallus M. R. Bakst U. S. Department of Agriculture Follow this and additional works at: https://digitalcommons.usu.edu/electron Part of the Biology Commons Recommended Citation Bakst, M. R. (1986) "Embryonic Development of the Chicken External Cloaca and Phallus," Scanning Electron Microscopy: Vol. 1986 : No. 2 , Article 35. Available at: https://digitalcommons.usu.edu/electron/vol1986/iss2/35 This Article is brought to you for free and open access by the Western Dairy Center at DigitalCommons@USU. It has been accepted for inclusion in Scanning Electron Microscopy by an authorized administrator of DigitalCommons@USU. For more information, please contact [email protected]. SCANNING ELECTRON MICROSCOPY /1986/11 (Pages 653-659) SEM Inc., AMF O'Hare (Chicago), IL 60666-0507 USA EMBRYONIC DEVELOPMENT OF THE CHICKEN EXTERNAL CLOACA AND PHALLUS M. R. Bakst U.S. Department of Agriculture Agricultural Research Service Avian Physiology Laboratory Beltsville, Maryland 20705 Phone: 301 344 2545 (Received for publication March 22, 1986: revised paper received May 23, 1986) Abstract Introduction The use of the scanning electron microscope In the course of investigating the mechanisms has provided new detailed information about the of erection, ejaculation, and semen dilution in the embryonic development of the chicken external chicken, it was found necessary to determine the cloaca and phallus and has cor:sequently clarified the embryonic origin of the structures in the cloaca origin of the differences between the anatomy of the which form the chicken Phallus nonprotrudens chicken and turkey phallus. -
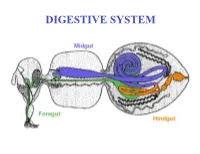
DIGESTIVE SYSTEM Generalized Insect Alimentary Tract the Digestive System Is Just a Tube Within a Surrounding Tube Called the Body
DIGESTIVE SYSTEM Generalized insect alimentary tract The digestive system is just a tube within a surrounding tube called the body. It starts with a mouth and ends with the anus. What goes on in between depends on the insect, its life stage and what it eats. The origin of the digestive tract. At the anterior pole of the embryo an indentation forms that will be the foregut or stomodeum. At the other end a similar thing occurs and the proctodeum or hindgut is formed. Both are lined by cuticle. They both are of ectodermal origin while the midgut is of mesodermal origin and is also called the mesenteron. This different origin of the midgut from the endoderm and not the ecotoderm probably explains why it is not lined with cuticle Anterior midgut invagination. In the bottom photo note the invagination starting forming the ventral furrow lumen (VF) MIDGUT FORMATION IN THE EMBRYO PMG in the above embryo shows the posterior midgut invagination cup where the posterior invagination shown in the drawing on the right will take place. Photo of Drosophila embryo. Hindgut invagination DIGESTIVE SYSTEM The digestive tract not only aids in obtaining, processing and digesting food molecules - It is the largest endocrine tissue in both humans and insects. The digestive system is involved in: 1. Obtaining food 2. Mechanically breaking it down into smaller particles that facilitate digestive enzymes acting on them 3. Enzymatic breakdown of larger food molecules into molecules that can pass through the digestive tract (midgut) and enter the hemolymph 4. Produces molecules or MESSENGERS (eg. -

Evidence for a Thoracic Crop in the Workers of Some Neotropical Pheidole Species (Formicidae: Myrmicinae)
Arthropod Structure & Development 59 (2020) 100977 Contents lists available at ScienceDirect Arthropod Structure & Development journal homepage: www.elsevier.com/locate/asd Evidence for a thoracic crop in the workers of some Neotropical Pheidole species (Formicidae: Myrmicinae) * A. Casadei-Ferreira a, , G. Fischer b, E.P. Economo b a Departamento de Zoologia, Universidade Federal do Parana, Avenida Francisco Heraclito dos Santos, s/n, Centro Politecnico, Curitiba, Mailbox 19020, CEP 81531-980, Brazil b Biodiversity and Biocomplexity Unit, Okinawa Institute of Science and Technology Graduate University, 1919-1 Tancha, Onna, Okinawa, 904-0495, Japan article info abstract Article history: The ability of ant colonies to transport, store, and distribute food resources through trophallaxis is a key Received 28 May 2020 advantage of social life. Nonetheless, how the structure of the digestive system has adapted across the Accepted 21 July 2020 ant phylogeny to facilitate these abilities is still not well understood. The crop and proventriculus, Available online xxx structures in the ant foregut (stomodeum), have received most attention for their roles in trophallaxis. However, potential roles of the esophagus have not been as well studied. Here, we report for the first Keywords: time the presence of an auxiliary thoracic crop in Pheidole aberrans and Pheidole deima using X-ray micro- Ants computed tomography and 3D segmentation. Additionally, we describe morphological modifications Dimorphism Mesosomal crop involving the endo- and exoskeleton that are associated with the presence of the thoracic crop. Our Liquid food results indicate that the presence of a thoracic crop in major workers suggests their potential role as Species group repletes or live food reservoirs, expanding the possibilities of tasks assumed by these individuals in the colony. -

Elixir Journal
45637 Ganesh Elumalai and Jenefa Princess / Elixir Embryology 103 (2017) 45637-45640 Available online at www.elixirpublishers.com (Elixir International Journal) Embryology Elixir Embryology 103 (2017) 45637-45640 “CLOACAL MEMBRANE ANOMALIES” EMBRYOLOGICAL BASIS AND ITS CLINICAL IMPORTANCE Ganesh Elumalai and Jenefa Princess Department of Embryology, College of Medicine, Texila American University, South America. ARTICLE INFO ABSTRACT Article history: Cloacal malformation is a rare but important anomaly. The cloacal anomaly is Received: 1 January 2017; characterised by the persistence of a common channel draining the urinary, genital and Received in revised form: alimentary tracts through a single orifice. It results from abnormal compartmentalization 1 February 2017; of features that are normal in the primitive female embryo. Abnormal embryology and Accepted: 10 February 2017; cloacal anatomy are described in detail. Cloacal abnormalities are usually diagnosed promptly in the neonatal period. Keywords © 2017 Elixir All rights reserved. Cloacal membrane, Uro-rectal septum, Extrophy of the cloaca, Recto-urinary fistulas, Anal agenesis, Rectal atresia. Introduction dilate them to make an anus.. Initial management focuses on Abnormal cloacal development takes place when rectum, anatomic remodelling of the urinary and gastrointestinal vagina and lower urinary tract fuse into a single common system to achieve continence. Improved paediatric channel. Persistent cloaca is a most severe malformation of management strategies have increased the patient survival into cloacal anomalies in girls and is associated with complex adult life. In order to provide appropriate advice, clinicians pelvic malformations. The abnormality of these structures who are undertaking life-long management of adolescent and varies from bladder neck to just beneath the perineal skin. -

Endoderm and Extraembryonic Structures
The Endoderm and Extraembryonic Structures Endoderm: Linings of a Tube • Divides into foregut, midgut, and hindgut • Openings to yolk sac are intestinal portals that close to middle to form yolk stalk Gut Regions How do the Ends Form? • Endodermal openings are stomodeum and proctodeum • Endoderm meets invagination of ectoderm What Comes from Foregut? • Foregut forms pharyngeal pouches, body tongue, thyroid, trachea, lung The Lungs • Lung develops by endothelial branching also typical of many glands • Depends on mesenchyme No mesenchyme--Mesenchyme What Comes from Foregut? • Pharyngeal region forms gills, eardrums, parathyroid, thymus • Breaks through to form gill slits with ectoderm • Connective tissue (cartilage) from neural crest The Pharynx Pouches and arches Further Down Liver and Pancreas • Linings from endoderm • Connective tissue from splanchnic mesoderm Amniotes Have Four Extra- embryonic “Membranes” • Amnion - maintains aqueous environment – amniote vertebrates • Chorion - gas exchange – in mammals --> placenta – also provides nutrition, hormones, immunity • Allantoic membrane - waste disposal/respiration – not necessary in humans because of placenta • Yolk Sac - nutrition – no yolk in humans (yolk sac holds primordial germ cells) Four “Membranes” Where do Membranes Originate? • Chorion and amnion from ectoderm and somatic mesoderm – = body wall or somatopleure • Allantois and yolk sac from endoderm and splanchnic mesoderm – = gut wall or splanchnopleure Extraembryonic Membranes • Membranous folds gradually separate embryo from the extraembryonic regions • Ectoderm + Mesoderm: – Amnion Somatopleure – Chorion (body wall) • Endoderm + Mesoderm: – Yolk sac Splanchnopleure – Allantois (gut wall) And More Folding The Caudal Region . -

High-Yield Embryology 4
LWBK356-FM_pi-xii.qxd 7/14/09 2:03 AM Page i Aptara Inc High-Yield TM Embryology FOURTH EDITION LWBK356-FM_pi-xii.qxd 7/14/09 2:03 AM Page ii Aptara Inc LWBK356-FM_pi-xii.qxd 7/14/09 2:03 AM Page iii Aptara Inc High-Yield TM Embryology FOURTH EDITION Ronald W. Dudek, PhD Professor Brody School of Medicine East Carolina University Department of Anatomy and Cell Biology Greenville, North Carolina LWBK356-FM_pi-xii.qxd 7/14/09 2:03 AM Page iv Aptara Inc Acquisitions Editor: Crystal Taylor Product Manager: Sirkka E. Howes Marketing Manager: Jennifer Kuklinski Vendor Manager: Bridgett Dougherty Manufacturing Manager: Margie Orzech Design Coordinator: Terry Mallon Compositor: Aptara, Inc. Copyright © 2010, 2007, 2001, 1996 Lippincott Williams & Wilkins, a Wolters Kluwer business. 351 West Camden Street 530 Walnut Street Baltimore, MD 21201 Philadelphia, PA 19106 Printed in China All rights reserved. This book is protected by copyright. No part of this book may be reproduced or transmitted in any form or by any means, including as photocopies or scanned-in or other electronic copies, or utilized by any information storage and retrieval system without written permission from the copyright owner, except for brief quotations embodied in critical articles and reviews. Materials appear- ing in this book prepared by individuals as part of their official duties as U.S. government employees are not covered by the above-mentioned copyright. To request permission, please contact Lippincott Williams & Wilkins at 530 Walnut Street, Philadelphia, PA 19106, via email at [email protected], or via website at lww.com (products and services). -

Structure, Function and Development of the Digestive System in Malacostracan Crustaceans and Adaptation to Different Lifestyles
Cell and Tissue Research (2019) 377:415–443 https://doi.org/10.1007/s00441-019-03056-0 REVIEW Structure, function and development of the digestive system in malacostracan crustaceans and adaptation to different lifestyles Jasna Štrus1 & Nada Žnidaršič1 & Polona Mrak1 & Urban Bogataj1 & Günter Vogt2 Received: 15 January 2019 /Accepted: 9 June 2019 /Published online: 4 July 2019 # Springer-Verlag GmbH Germany, part of Springer Nature 2019 Abstract The digestive system of the malacostracan crustaceans, namely the decapods, isopods, amphipods and mysids, is among the most complex organ systems of the animal kingdom serving multiple functions such as food processing, absorption and storage of nutrients, synthesis of digestive enzymes and blood proteins, detoxification of xenobiotics and osmoregulation. It is rather well investigated compared to other invertebrates because the Malacostraca include many ecological keystone species and food items for humans. The Decapoda and Peracarida share food processing with chewing and filtering structures of the stomach but differ with respect to morphology and ultrastructure of the digestive glands. In the Peracarida, the digestive glands are composed of few, relatively large lateral caeca, whereas in the Decapoda, hundreds to thousands of blindly ending tubules form a voluminous hepatopancreas. Morphogenesis and onset of functionality of the digestive system strongly depend on the mode of development. The digestive system is early developed in species with feeding planktonic larvae and appears late in species with direct lecithotrophic development. Some structures of the digestive system like the stomach ossicles are rather constant in higher taxa and are of taxonomic value, whereas others like the chewing structures are to some degree adapted to the feeding strategy. -

Embryology20 Dr.Ban
Embryology20 Dr.Ban The midgut Organs in the adult mid gut: Duodenum Jejunum Ileum Cecum Appendix Ascending colon Hepatic flexure of colon Transverse colon (proximal 2/3rd ) The mid gut is the portion of the embryo from which most of the intestine develop. During development, the human mid gut undergoes a rapid phase of growth in which the loop of mid gut ( U shaped loop )herniates outside of the abdominal cavity of the fetus and protrudes into the umbilical cord. This herniation is physiological (occurs normally). • The upper limb of the U is destined to be form the future small intestine • The lower limb forms the ascending and transverse colon. • At the tip of the U, the mid gut is attached to the umbilicus by a thin duct called the vitellointestinal duct which disappears during the later stages of development. • The space between the 2 limbs of the U has the mesentry – a fan shaped structure that holds all the loops of intestine together. 1 Embryology20 Dr.Ban The midgut loops slips back out of the umbilical cord and the physiological hernia ceases to exist. This change coincides with : the termination of the yolk sac and the counter clockwise rotation of the two limbs of the midgut loop around their combined central axis. The U loop undergoes 3 rotations in a step wise manner: First it rotates by 90° in the anticlockwise direction (as seen from the front) along the axis of the superior mesentric artery. At the end of this first rotation the upper limb of the U, or the future ileum comes to lie on the fetus’s right and the lower limb of U or the future colon lies on the left.At the end of 10th week, the midgut retracts back into the abdominal cavity. -

Control of Gut Development by Fork Head and Cell Signaling Molecules in Drosophila
ELSEVIER Mechanisms of Development 58 (1996) 3-14 Control of gut development by fork head and cell signaling molecules in Drosophila Michael Hoch*, Michael J. Pankratz Mar-Plunck-lnstitut,~r Biophysikalische Chemie, Abteilung Molekulare Entwicklungsbiologie, Am Fassberg. 37077 Gdttingen, Germany Received 14 March 1996; accepted 9 April 1996 Abstract The alimentary canal of most animals can be subdivided into a fore-, mid- and hindgut portion, each gut part possessing distinct physiological functions. The genetic basis underlying the formation of the different gut parts is poorly understood. Here we show that the Drosophila genes hedgehog, wingless and decapentuplegic, which encode cell signaling molecules, are required for the establish- ment of signaling centers that coordinate morphogenesis in the hindgut epithelium. The activation of these genes in the developing as well as in the foregut requires fork head, which encodes a transcription factor. Furthermore, we demonstrate that hedgehog and win- gless activities in the gut epithelial cells are required for the expression of the homeobox gene bagpipe in the ensheathing visceral mesoderm. These results provide strong evidence that similar principles underlie Drosophila fore- and hindgut development, and that the genetic hierarchy of gut development might be conserved between Drosophila and vertebrates. Keywords: Cell signaling in the gut; hedgehog; wingless; decapentaplegic; fork head; bagpipe; Gut; Morphogenesis 1. Introduction a continuous gut tube is generated. Regional specification then occurs and several gut-associated organs are formed Nutrition and hydration are basic needs of all organ- at the junction between the different gut parts. isms. The organ which is required to fulfill these needs in The genetic basis underlying gut development is only animals is the gut, which most likely belongs to the most poorly understood. -
Development of the Gastrointestinal Tract and the Urogenital System and Malformations
Development of the gastrointestinal tract and the urogenital system and malformations for pharmacists Semmelweis University, Department of Anatomy, Histology and Embryology 2017. 04.03. by Krisztina H.-Minkó Folding of the Embryo (cross section) https://www.youtube.com/watch?v=qMnpxP6EeIY 4th week Folding of the Embryo (Longitudinal section) Hindgut Foregut Posterior opening of Anterior opening Caudal gut gut Notochord of gut Cranial gut Body stalk Midgut Vitelline duct Allantois Stomodeum + Cloacal Heart anlage membrane buccopharyngeal membrane Folding of the Embryo (Intraembryonic Mesoderm) Dorsal aorta Mesonephros Dorsal mesogastrium Intraembryonal coelom Somites Gut tube Neural tube Visceral mesoderm Notochord Ventral Dorsal aorta mesogastrium Mesonephros Genital crest Gut tube Coelom Intraembryonal coelom Ventral mesentery Dorsal mesentery The endodermal gut tube created by body folding during the 4th week consists of a blindended cranial foregut, a Primitive Gut Tube blind-ended caudal hindgut, and a midgut open to the yolk sac through the vitelline duct. Pharyngeal gut Buccopharyngeal Thyreoglossal membrane duct Yolk sac Esophagus Foregut Heart Lung Vitelline duct Stomach Duodenojejunal Body stalk flexure Midgut Caudal gut Cecum Left colic Cloacal membrane flexure Allantois Cloaca Hindgut Development of GI tract https://www.youtube.com/watch?v=tx3Cn8g-_e0 Vascularisation of the primitive gut tube The arterial supply to the gut develops through consolidation and reduction of the ventral branches of the dorsal aortae that anastomose with the vessel plexuses originally supplying blood to the yolk sac. About five of these vitelline artery derivatives vascularize the thoracic foregut, and three—the celiac, superior mesenteric, and inferior mesenteric arteries—vascularize the abdominal gut. By convention, the boundaries of the foregut, midgut, and hindgut portions of the abdominal gut tube are determined by the respective territories of these three arteries.