Structure and Alpha Biodiversity of Major Plant Communities in Chile, a Distant Gondwanan Relation of Australia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Chapter14.Pdf
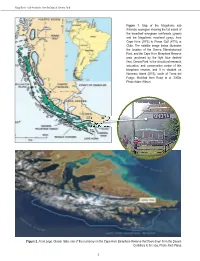
PART I • Omora Park Long-Term Ornithological Research Program THE OMORA PARK LONG-TERM ORNITHOLOGICAL RESEARCH PROGRAM: 1 STUDY SITES AND METHODS RICARDO ROZZI, JAIME E. JIMÉNEZ, FRANCISCA MASSARDO, JUAN CARLOS TORRES-MURA, AND RAJAN RIJAL In January 2000, we initiated a Long-term Ornithological Research Program at Omora Ethnobotanical Park in the world's southernmost forests: the sub-Antarctic forests of the Cape Horn Biosphere Reserve. In this chapter, we first present some key climatic, geographical, and ecological attributes of the Magellanic sub-Antarctic ecoregion compared to subpolar regions of the Northern Hemisphere. We then describe the study sites at Omora Park and other locations on Navarino Island and in the Cape Horn Biosphere Reserve. Finally, we describe the methods, including censuses, and present data for each of the bird species caught in mist nets during the first eleven years (January 2000 to December 2010) of the Omora Park Long-Term Ornithological Research Program. THE MAGELLANIC SUB-ANTARCTIC ECOREGION The contrast between the southwestern end of South America and the subpolar zone of the Northern Hemisphere allows us to more clearly distinguish and appreciate the peculiarities of an ecoregion that until recently remained invisible to the world of science and also for the political administration of Chile. So much so, that this austral region lacked a proper name, and it was generally subsumed under the generic name of Patagonia. For this reason, to distinguish it from Patagonia and from sub-Arctic regions, in the early 2000s we coined the name “Magellanic sub-Antarctic ecoregion” (Rozzi 2002). The Magellanic sub-Antarctic ecoregion extends along the southwestern margin of South America between the Gulf of Penas (47ºS) and Horn Island (56ºS) (Figure 1). -

Bioclimatic and Phytosociological Diagnosis of the Species of the Nothofagus Genus (Nothofagaceae) in South America
International Journal of Geobotanical Research, Vol. nº 1, December 2011, pp. 1-20 Bioclimatic and phytosociological diagnosis of the species of the Nothofagus genus (Nothofagaceae) in South America Javier AMIGO(1) & Manuel A. RODRÍGUEZ-GUITIÁN(2) (1) Laboratorio de Botánica, Facultad de Farmacia, Universidad de Santiago de Compostela (USC). E-15782 Santiago de Com- postela (Galicia, España). Phone: 34-881 814977. E-mail: [email protected] (2) Departamento de Producción Vexetal. Escola Politécnica Superior de Lugo-USC. 27002-Lugo (Galicia, España). E-mail: [email protected] Abstract The Nothofagus genus comprises 10 species recorded in the South American subcontinent. All are important tree species in the ex- tratropical, Mediterranean, temperate and boreal forests of Chile and Argentina. This paper presents a summary of data on the phyto- coenotical behaviour of these species and relates the plant communities to the measurable or inferable thermoclimatic and ombrocli- matic conditions which affect them. Our aim is to update the phytosociological knowledge of the South American temperate forests and to assess their suitability as climatic bioindicators by analysing the behaviour of those species belonging to their most represen- tative genus. Keywords: Argentina, boreal forests, Chile, mediterranean forests, temperate forests. Introduction tually give rise to a temperate territory with rainfall rates as high as those of regions with a Tropical pluvial bio- The South American subcontinent is usually associa- climate; iii. finally, towards the apex of the American ted with a tropical environment because this is in fact the Southern Cone, this temperate territory progressively dominant bioclimatic profile from Panamá to the north of gives way to a strip of land with a Boreal bioclimate. -

New Plantings in the Arboretum the YEAR in REVIEW
Four new have been Four new Yoshino cherry trees have Yoshinobeen planted along planted Azalea Way. cherry trees along Azalea New Plantings in the Arboretum THE YEAR IN REVIEW T EX T B Y R AY L A R SON P HO T OS B Y N IA ll D UNNE n the five years that I have been curator, 2018 was the most active in terms of new plantings in the Arboretum. A majority of these centered around the new Arboretum Loop Trail and adjacent areas, many of which were enhanced, rehabilitated and Iaugmented. We also made improvements to a few other collection and garden areas with individual and smaller plantings. Following is a summary of some of the more noticeable new plantings you might encounter during your next visit. Winter 2019 v 3 Arboretum Entrance Perhaps the most obvious major planting occurred in March, just north of the Graham Visitors Center, with the creation of a new, large bed at the southeast corner of the intersection of Arboretum Drive and Foster Island Road. This intersection changed a lot as part of the Loop Trail construction—with the addition of new curbs and crosswalks—and we wanted to create a fitting entrance to the Arboretum at its north end. The new planting was also intended to alleviate some of the soil compaction and social trails that had developed on the east side of Arboretum Drive during trail construction. What’s more, we wanted to encourage pedes- trians to use the new gravel trail on the west side of the Drive to connect from the lower parking lots to the Visitors Center—rather than walk in the road. -

A Chronology of Middle Missouri Plains Village Sites
Smithsonian Institution Scholarly Press smithsonian contributions to botany • n u m b e r 9 2 Smithsonian Institution Scholarly Press TaxonomicA Chronology Revision of of the MiddleChiliotrichum Missouri Group Plains Villagesensu stricto Sites (Compositae: Astereae) By Craig M. Johnson Joséwith Mauricio contributions Bonifacino by Stanley A. Ahler, Herbert Haas, and Georges Bonani SERIES PUBLICATIONS OF THE SMITHSONIAN INSTITUTION Emphasis upon publication as a means of “diffusing knowledge” was expressed by the first Secretary of the Smithsonian. In his formal plan for the Institution, Joseph Henry outlined a program that included the following statement: “It is proposed to publish a series of reports, giving an account of the new discoveries in science, and of the changes made from year to year in all branches of knowledge.” This theme of basic research has been adhered to through the years by thousands of titles issued in series publications under the Smithsonian imprint, com- mencing with Smithsonian Contributions to Knowledge in 1848 and continuing with the following active series: Smithsonian Contributions to Anthropology Smithsonian Contributions to Botany Smithsonian Contributions in History and Technology Smithsonian Contributions to the Marine Sciences Smithsonian Contributions to Museum Conservation Smithsonian Contributions to Paleobiology Smithsonian Contributions to Zoology In these series, the Institution publishes small papers and full-scale monographs that report on the research and collections of its various museums and bureaus. The Smithsonian Contributions Series are distributed via mailing lists to libraries, universities, and similar institu- tions throughout the world. Manuscripts submitted for series publication are received by the Smithsonian Institution Scholarly Press from authors with direct affilia- tion with the various Smithsonian museums or bureaus and are subject to peer review and review for compliance with manuscript preparation guidelines. -

Impact of Extreme Weather Events on Aboveground Net Primary Productivity and Sheep Production in the Magellan Region, Southernmost Chilean Patagonia
geosciences Article Impact of Extreme Weather Events on Aboveground Net Primary Productivity and Sheep Production in the Magellan Region, Southernmost Chilean Patagonia Pamela Soto-Rogel 1,* , Juan-Carlos Aravena 2, Wolfgang Jens-Henrik Meier 1, Pamela Gross 3, Claudio Pérez 4, Álvaro González-Reyes 5 and Jussi Griessinger 1 1 Institute of Geography, Friedrich–Alexander-University of Erlangen–Nürnberg, 91054 Erlangen, Germany; [email protected] (W.J.-H.M.); [email protected] (J.G.) 2 Centro de Investigación Gaia Antártica, Universidad de Magallanes, Punta Arenas 6200000, Chile; [email protected] 3 Servicio Agrícola y Ganadero (SAG), Punta Arenas 6200000, Chile; [email protected] 4 Private Consultant, Punta Arenas 6200000, Chile; [email protected] 5 Hémera Centro de Observación de la Tierra, Escuela de Ingeniería Forestal, Facultad de Ciencias, Universidad Mayor, Camino La Pirámide 5750, Huechuraba, Santiago 8580745, Chile; [email protected] * Correspondence: [email protected] Received: 28 June 2020; Accepted: 13 August 2020; Published: 16 August 2020 Abstract: Spatio-temporal patterns of climatic variability have effects on the environmental conditions of a given land territory and consequently determine the evolution of its productive activities. One of the most direct ways to evaluate this relationship is to measure the condition of the vegetation cover and land-use information. In southernmost South America there is a limited number of long-term studies on these matters, an incomplete network of weather stations and almost no database on ecosystems productivity. In the present work, we characterized the climate variability of the Magellan Region, southernmost Chilean Patagonia, for the last 34 years, studying key variables associated with one of its main economic sectors, sheep production, and evaluating the effect of extreme weather events on ecosystem productivity and sheep production. -

Museum of Economic Botany, Kew. Specimens Distributed 1901 - 1990
Museum of Economic Botany, Kew. Specimens distributed 1901 - 1990 Page 1 - https://biodiversitylibrary.org/page/57407494 15 July 1901 Dr T Johnson FLS, Science and Art Museum, Dublin Two cases containing the following:- Ackd 20.7.01 1. Wood of Chloroxylon swietenia, Godaveri (2 pieces) Paris Exibition 1900 2. Wood of Chloroxylon swietenia, Godaveri (2 pieces) Paris Exibition 1900 3. Wood of Melia indica, Anantapur, Paris Exhibition 1900 4. Wood of Anogeissus acuminata, Ganjam, Paris Exhibition 1900 5. Wood of Xylia dolabriformis, Godaveri, Paris Exhibition 1900 6. Wood of Pterocarpus Marsupium, Kistna, Paris Exhibition 1900 7. Wood of Lagerstremia parviflora, Godaveri, Paris Exhibition 1900 8. Wood of Anogeissus latifolia , Godaveri, Paris Exhibition 1900 9. Wood of Gyrocarpus jacquini, Kistna, Paris Exhibition 1900 10. Wood of Acrocarpus fraxinifolium, Nilgiris, Paris Exhibition 1900 11. Wood of Ulmus integrifolia, Nilgiris, Paris Exhibition 1900 12. Wood of Phyllanthus emblica, Assam, Paris Exhibition 1900 13. Wood of Adina cordifolia, Godaveri, Paris Exhibition 1900 14. Wood of Melia indica, Anantapur, Paris Exhibition 1900 15. Wood of Cedrela toona, Nilgiris, Paris Exhibition 1900 16. Wood of Premna bengalensis, Assam, Paris Exhibition 1900 17. Wood of Artocarpus chaplasha, Assam, Paris Exhibition 1900 18. Wood of Artocarpus integrifolia, Nilgiris, Paris Exhibition 1900 19. Wood of Ulmus wallichiana, N. India, Paris Exhibition 1900 20. Wood of Diospyros kurzii , India, Paris Exhibition 1900 21. Wood of Hardwickia binata, Kistna, Paris Exhibition 1900 22. Flowers of Heterotheca inuloides, Mexico, Paris Exhibition 1900 23. Leaves of Datura Stramonium, Paris Exhibition 1900 24. Plant of Mentha viridis, Paris Exhibition 1900 25. Plant of Monsonia ovata, S. -

Chile: a Journey to the End of the World in Search of Temperate Rainforest Giants
Eliot Barden Kew Diploma Course 53 July 2017 Chile: A Journey to the end of the world in search of Temperate Rainforest Giants Valdivian Rainforest at Alerce Andino Author May 2017 1 Eliot Barden Kew Diploma Course 53 July 2017 Table of Contents 1. Title Page 2. Contents 3. Table of Figures/Introduction 4. Introduction Continued 5. Introduction Continued 6. Aims 7. Aims Continued / Itinerary 8. Itinerary Continued / Objective / the Santiago Metropolitan Park 9. The Santiago Metropolitan Park Continued 10. The Santiago Metropolitan Park Continued 11. Jardín Botánico Chagual / Jardin Botanico Nacional, Viña del Mar 12. Jardin Botanico Nacional Viña del Mar Continued 13. Jardin Botanico Nacional Viña del Mar Continued 14. Jardin Botanico Nacional Viña del Mar Continued / La Campana National Park 15. La Campana National Park Continued / Huilo Huilo Biological Reserve Valdivian Temperate Rainforest 16. Huilo Huilo Biological Reserve Valdivian Temperate Rainforest Continued 17. Huilo Huilo Biological Reserve Valdivian Temperate Rainforest Continued 18. Huilo Huilo Biological Reserve Valdivian Temperate Rainforest Continued / Volcano Osorno 19. Volcano Osorno Continued / Vicente Perez Rosales National Park 20. Vicente Perez Rosales National Park Continued / Alerce Andino National Park 21. Alerce Andino National Park Continued 22. Francisco Coloane Marine Park 23. Francisco Coloane Marine Park Continued 24. Francisco Coloane Marine Park Continued / Outcomes 25. Expenditure / Thank you 2 Eliot Barden Kew Diploma Course 53 July 2017 Table of Figures Figure 1.) Valdivian Temperate Rainforest Alerce Andino [Photograph; Author] May (2017) Figure 2. Map of National parks of Chile Figure 3. Map of Chile Figure 4. Santiago Metropolitan Park [Photograph; Author] May (2017) Figure 5. -

Flora Vascular De Un Remanente De Bosque Esclerófilo Mediterráneo Costero: Estación De Biología Terrestre De Hualpén, Región Del Biobío, Chile
Gayana Bot. 75(1):75(1), 2018466-481, 2018. ISSN 0016-5301 Artículo Original Flora vascular de un remanente de bosque esclerófilo mediterráneo costero: Estación de Biología Terrestre de Hualpén, Región del Biobío, Chile Vascular flora of a remnant of coastal Mediterranean Sclerophyll forest: Hualpén Terrestrial Biology Station, Biobío Region, Chile MARÍA MORENO-CHACÓN1, DANIELA MARDONES2, NATALY VIVEROS1*, KARINA MADRIAZA1, FERNANDO CARRASCO-URRA1, ALICIA MARTICORENA1, CARLOS BAEZA1, ROBERTO RODRÍGUEZ1 & ALFREDO SALDAÑA1 1Departamento de Botánica, Facultad de Ciencias Naturales y Oceanográficas, Universidad de Concepción, Casilla 160-C, Concepción, Chile. 2Departamento de Ciencias Ecológicas, Facultad de Ciencias, Universidad de Chile. *[email protected] RESUMEN Los remanentes de los bosques esclerófilos mediterráneos costeros en Chile tienen una baja representatividad en el Sistema Nacional de Áreas Silvestres Protegidas, a pesar de su alto endemismo y riqueza de especies. En la Región del Biobío, la Estación de Biología Terrestre de Hualpén (EBT) conserva un remanente de este tipo de bosque, para el cual no existen revisiones que describan su flora, lo que dificulta la formulación y el desarrollo de estrategias para su protección y conservación. Este trabajo tiene como objetivos: a) describir la composición taxonómica de las plantas vasculares de la ETB y b) entregar información sobre su origen geográfico, hábito y estado de conservación. El listado florístico obtenido incluye tanto las especies determinadas a través del muestreo sistemático en el área de estudio durante un año, así como las especies de la EBT ingresadas en la colección del Herbario de la Universidad de Concepción. Se determinaron 294 especies de plantas vasculares de las cuales 71 son endémicas, 124 son nativas y 99 son introducidas. -

Bosque Pehuén Park's Flora: a Contribution to the Knowledge of the Andean Montane Forests in the Araucanía Region, Chile Author(S): Daniela Mellado-Mansilla, Iván A
Bosque Pehuén Park's Flora: A Contribution to the Knowledge of the Andean Montane Forests in the Araucanía Region, Chile Author(s): Daniela Mellado-Mansilla, Iván A. Díaz, Javier Godoy-Güinao, Gabriel Ortega-Solís and Ricardo Moreno-Gonzalez Source: Natural Areas Journal, 38(4):298-311. Published By: Natural Areas Association https://doi.org/10.3375/043.038.0410 URL: http://www.bioone.org/doi/full/10.3375/043.038.0410 BioOne (www.bioone.org) is a nonprofit, online aggregation of core research in the biological, ecological, and environmental sciences. BioOne provides a sustainable online platform for over 170 journals and books published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/page/terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and non-commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. R E S E A R C H A R T I C L E ABSTRACT: In Chile, most protected areas are located in the southern Andes, in mountainous land- scapes at mid or high altitudes. Despite the increasing proportion of protected areas, few have detailed inventories of their biodiversity. This information is essential to define threats and develop long-term • integrated conservation programs to face the effects of global change. -

Native Plant Diversity and Composition Across a Pinus Radiata D.Don Plantation Landscape in South-Central Chile—The Impact Of
Article Native Plant Diversity and Composition Across a Pinus radiata D.Don Plantation Landscape in South-Central Chile—The Impact of Plantation Age, Logging Roads and Alien Species Steffi Heinrichs 1,*, Aníbal Pauchard 2,3 and Peter Schall 1 1 Department Silviculture and Forest Ecology of the Temperate Zones, University of Goettingen, 37077 Göttingen, Germany; [email protected] 2 Facultad de Ciencias Forestales, Universidad de Concepción, Concepción 3349001, Chile; [email protected] 3 Institute of Ecology and Biodiversity (IEB), Santiago 8320000, Chile * Correspondence: [email protected]; Tel.: +49-551-395-974 Received: 18 July 2018; Accepted: 12 September 2018; Published: 14 September 2018 Abstract: Alien tree plantations are expanding globally with potential negative effects for native biodiversity. We investigated plant species diversity and composition in a Pinus radiata landscape in south-central Chile, a biodiversity hotspot, by sampling understory vegetation in different plantation age classes, along forest roads and in natural forest remnants in order to find effective conservation measures for native biodiversity. Plantations, including different age classes and roadsides, maintained high native species richness at the landscape scale but supported a completely different community composition than natural forests. Thus, natural forest remnants must be conserved as plantations cannot replace them. Certain natural forest species occurred frequently in mature plantations and can represent starting points for retaining natural elements in plantations. Generalist native and alien species benefited from plantation management, mainly in young plantations and along roadsides. Stand maturation and a closed canopy, though, reduced alien species occurrences within plantations. Along roads, shade-tolerant aliens should be monitored and removed as they can potentially invade natural forests. -

Shoot Development and Dieback in Progenies of Nothofagus Obliqua Javier G
Shoot development and dieback in progenies of Nothofagus obliqua Javier G. Puntieri, Javier E. Grosfeld, Marina Stecconi, Cecilia Brion, María Marta Azpilicueta, Leonardo Gallo, Daniel Barthélémy To cite this version: Javier G. Puntieri, Javier E. Grosfeld, Marina Stecconi, Cecilia Brion, María Marta Azpilicueta, et al.. Shoot development and dieback in progenies of Nothofagus obliqua. Annals of Forest Science, Springer Nature (since 2011)/EDP Science (until 2010), 2007, 64 (8), pp.839-844. 10.1051/forest:2007068. hal-00259241 HAL Id: hal-00259241 https://hal.archives-ouvertes.fr/hal-00259241 Submitted on 30 May 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Copyright Ann. For. Sci. 64 (2007) 839–844 Available online at: c INRA, EDP Sciences, 2007 www.afs-journal.org DOI: 10.1051/forest:2007068 Original article Shoot development and dieback in progenies of Nothofagus obliqua Javier Puntieria,b*,JavierGrosfelda,b,MarinaStecconib, Cecilia Briona, María Marta Azpilicuetac, Leonardo Galloc,DanielBarthel´ emy´ d a Departamento de Botánica, Universidad Nacional del Comahue, Quintral 1250, 8400, Bariloche, Argentina b Consejo Nacional de Investigaciones Científicas y Técnicas, Argentina c Laboratorio de Genética Forestal, Instituto Nacional de Tecnología Agropecuaria, EEA Bariloche, Argentina d INRA, Unité Mixte de Recherche CIRAD-CNRS-INRA-IRD-Université Montpellier 2, “ botAnique et bioinforMatique de l’Architecture des Plantes ” (AMAP), UMR T51 (CIRAD), UMR 5120 (CNRS), UMR 931 (INRA), M123 (IRD), UM27 (UMII) TA A-51/PS2, Blvd. -

Plan De Manejo Parque Nacional Fray Jorge
REPUBLICA DE CHILE MINISTERIO DE AGRICULTURA CORPORACION NACIONAL FORESTAL IV REGION - COQUIMBO DOCUMENTO DE TRABAJO N° 297 Este documento se presta a domicilio, con préstamo interbibliotecario , solo el día VIERNES a las 16 :30 horas , para ser devuelto el día LUNES a las 9 :30 horas. ^5 Resolución N° 372 Mat.: Apruébase Plan de Manejo Parque Nacional Bosque Fray Jorge. Santiago, 22 de Diciembre de 1998 VISTOS Las facultades que me confiere el articulo 20, letras a) y g) de los Estatutos de la Corporación y el articulo 19, letra «g» de su Reglamento Orgánico; lo establecido en la Resolución 200 del 11 de Julio de 1983, de esta Dirección Ejecutiva; y CONSIDERANDO: Que por Decreto Supremo N° 867 del Ministerio de Bienes Nacionales del 30 de Diciembre de 1981, se fusionan los Parques Nacionales Bosque Fray Jorge, Talinay y Punta del Viento, creando como una sola unidad el Parque Nacional Bosque Fray Jorge. Que la Corporación Nacional Forestal es el organismo encargado de la tuición y administración del Parque antes referido. Que para alcanzar los objetivos que con la creación de tales unidades territoriales se persigue, es indis- pensable planificar las actividades a realizar en ellas, as¡ como las normas que regularán el uso y apro- vechamiento del Parque Nacional a través de un Plan de Manejo. RESUELVO: PRIMERO: Apruébase el Plan de Manejo del Parque Nacional Bosque Fray Jorge, en cuya elaboración participaron los siguientes profesionales: Como responsables técnicos de CONAF IV Región Srs. Marcos Cordero V., Jefe U.G. Patrimonio Silvestre Regional, Víctor Lagos, Jefe Unidad Técnica U.G.