Glutamatergic Signaling Along the Microbiota-Gut-Brain Axis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

From Human Emotions to Robot Emotions
1 American Association for Artificial Intelligence – Spring Symposium 3/2004, Stanford University – Keynote Lecture. From Human Emotions to Robot Emotions Jean-Marc Fellous The Salk Institute for Neurobiological Studies 10010 N. Torrey Pines Road, la Jolla, CA 92037 [email protected] Abstract1 open a new window on the neural bases of emotions that may offer new ways of thinking about implementing robot- The main difficulties that researchers face in understanding emotions. emotions are difficulties only because of the narrow- mindedness of our views on emotions. We are not able to Why are emotions so difficult to study? free ourselves from the notion that emotions are necessarily human emotions. I will argue that if animals have A difficulty in studying human emotions is that here are emotions, then so can robots. Studies in neuroscience have significant individual differences, based on experiential as shown that animal models, though having limitations, have well as genetic factors (Rolls, 1998; Ortony, 2002; significantly contributed to our understanding of the Davidson, 2003a, b; Ortony et al., 2004). My fear at the functional and mechanistic aspects of emotions. I will sight of a bear may be very different from the fear suggest that one of the main functions of emotions is to experienced by a park-ranger who has a better sense for achieve the multi-level communication of simplified but high impact information. The way this function is achieved bear-danger and knows how to react. My fear might also be in the brain depends on the species, and on the specific different from that of another individual who has had about emotion considered. -

A Guide to Glutamate Receptors
A guide to glutamate receptors 1 Contents Glutamate receptors . 4 Ionotropic glutamate receptors . 4 - Structure ........................................................................................................... 4 - Function ............................................................................................................ 5 - AMPA receptors ................................................................................................. 6 - NMDA receptors ................................................................................................. 6 - Kainate receptors ............................................................................................... 6 Metabotropic glutamate receptors . 8 - Structure ........................................................................................................... 8 - Function ............................................................................................................ 9 - Group I: mGlu1 and mGlu5. .9 - Group II: mGlu2 and mGlu3 ................................................................................. 10 - Group III: mGlu4, mGlu6, mGlu7 and mGlu8 ............................................................ 10 Protocols and webinars . 11 - Protocols ......................................................................................................... 11 - Webinars ......................................................................................................... 12 References and further reading . 13 Excitatory synapse pathway -

S Efficacy in Treating Bipolar Depression: a Longitudinal Proton Magnetic Resonance Spectroscopy Study
Neuropsychopharmacology (2009) 34, 1810–1818 & 2009 Nature Publishing Group All rights reserved 0893-133X/09 $32.00 www.neuropsychopharmacology.org Decreased Glutamate/Glutamine Levels May Mediate Cytidine’s Efficacy in Treating Bipolar Depression: A Longitudinal Proton Magnetic Resonance Spectroscopy Study Sujung J Yoon*,1, In Kyoon Lyoo2,3, Charlotte Haws4,5, Tae-Suk Kim1, Bruce M Cohen2,6 and 4,5 Perry F Renshaw 1Department of Psychiatry, Catholic University College of Medicine, Seoul, South Korea; 2Department of Psychiatry, Harvard Medical School, Boston, MA, USA; 3Brain Imaging Center and Clinical Research Center, Seoul National University Hospital, Seoul, South Korea; 4Department of 5 Psychiatry, The Brain Institute, University of Utah, Salt Lake City, UT, USA; Department of Veterans Affairs VISN 19 MIRECC, Salt Lake City, UT, 6 USA; Molecular Pharmacology Laboratory, Harvard Medical School, McLean Hospital, Belmont, MA, USA Targeting the glutamatergic system has been suggested as a promising new option for developing treatment strategies for bipolar depression. Cytidine, a pyrimidine, may exert therapeutic effects through a pathway that leads to altered neuronal-glial glutamate cycling. Pyrimidines are also known to exert beneficial effects on cerebral phospholipid metabolism, catecholamine synthesis, and mitochondrial function, which have each been linked to the pathophysiology of bipolar depression. This study was aimed at determining cytidine’s efficacy in bipolar depression and at assessing the longitudinal effects of cytidine on cerebral glutamate/glutamine levels. Thirty-five patients with bipolar depression were randomly assigned to receive the mood-stabilizing drug valproate plus either cytidine or placebo for 12 weeks. Midfrontal cerebral glutamate/glutamine levels were measured using proton magnetic resonance spectroscopy before and after 2, 4, and 12 weeks of oral cytidine administration. -

What Can Be Learned from White Matter Alterations in Antisocial Girls Willeke M
Menks WM, Raschle NM. J Neurol Neuromedicine (2017) 2(7): 16-20 Neuromedicine www.jneurology.com www.jneurology.com Journal of Neurology & Neuromedicine Mini Review Open Access What can be learned from white matter alterations in antisocial girls Willeke M. Menks1, Christina Stadler1 and Nora M. Raschle1 1Department of Child and Adolescent Psychiatry, University of Basel, Psychiatric University Hospital Basel, Switzerland. Article Info ABSTRACT Article Notes Antisocial behavior in youths constitutes a major public health problem Received: June 17, 2017 worldwide. Conduct disorder is a severe variant of antisocial behavior with higher Accepted: July 31, 2017 prevalence rates for boys (12%) as opposed to girls (7%). A better understanding *Correspondence: of the underlying neurobiological mechanisms of conduct disorder is warranted Dr. Willeke Menks, PhD to improve identification, diagnosis, or treatment. Functional and structural Department of Child and Adolescent Psychiatry (KJPK), neuroimaging studies have indicated several key brain regions within the limbic Psychiatric University Clinics Basel (UPK) system and prefrontal cortex that are altered in youths with conduct disorder. Schanzenstrasse 13, CH-4056 Basel, Switzerland Examining the structural connectivity, i.e. white matter fiber tracts connecting Tel. +41 61 265 89 76 these brain areas, may further inform about the underlying neural mechanisms. Fax +41 61 265 89 61 Diffusion tensor imaging (DTI) is a non-invasive technique that can evaluate the © 2017 Menks WM & Raschle NM. This article is distributed white matter integrity of fiber tracts throughout the brain. To date, DTI studies have under the terms of the Creative Commons Attribution 4.0 found several white matter tracts that are altered in youths with conduct disorder. -

Metabotropic Glutamate Receptors
mGluR Metabotropic glutamate receptors mGluR (metabotropic glutamate receptor) is a type of glutamate receptor that are active through an indirect metabotropic process. They are members of thegroup C family of G-protein-coupled receptors, or GPCRs. Like all glutamate receptors, mGluRs bind with glutamate, an amino acid that functions as an excitatoryneurotransmitter. The mGluRs perform a variety of functions in the central and peripheral nervous systems: mGluRs are involved in learning, memory, anxiety, and the perception of pain. mGluRs are found in pre- and postsynaptic neurons in synapses of the hippocampus, cerebellum, and the cerebral cortex, as well as other parts of the brain and in peripheral tissues. Eight different types of mGluRs, labeled mGluR1 to mGluR8, are divided into groups I, II, and III. Receptor types are grouped based on receptor structure and physiological activity. www.MedChemExpress.com 1 mGluR Agonists, Antagonists, Inhibitors, Modulators & Activators (-)-Camphoric acid (1R,2S)-VU0155041 Cat. No.: HY-122808 Cat. No.: HY-14417A (-)-Camphoric acid is the less active enantiomer (1R,2S)-VU0155041, Cis regioisomer of VU0155041, is of Camphoric acid. Camphoric acid stimulates a partial mGluR4 agonist with an EC50 of 2.35 osteoblast differentiation and induces μM. glutamate receptor expression. Camphoric acid also significantly induced the activation of NF-κB and AP-1. Purity: ≥98.0% Purity: ≥98.0% Clinical Data: No Development Reported Clinical Data: No Development Reported Size: 10 mM × 1 mL, 100 mg Size: 10 mM × 1 mL, 5 mg, 10 mg, 25 mg (2R,4R)-APDC (R)-ADX-47273 Cat. No.: HY-102091 Cat. No.: HY-13058B (2R,4R)-APDC is a selective group II metabotropic (R)-ADX-47273 is a potent mGluR5 positive glutamate receptors (mGluRs) agonist. -

The G Protein-Coupled Glutamate Receptors As Novel Molecular Targets in Schizophrenia Treatment— a Narrative Review
Journal of Clinical Medicine Review The G Protein-Coupled Glutamate Receptors as Novel Molecular Targets in Schizophrenia Treatment— A Narrative Review Waldemar Kryszkowski 1 and Tomasz Boczek 2,* 1 General Psychiatric Ward, Babinski Memorial Hospital in Lodz, 91229 Lodz, Poland; [email protected] 2 Department of Molecular Neurochemistry, Medical University of Lodz, 92215 Lodz, Poland * Correspondence: [email protected] Abstract: Schizophrenia is a severe neuropsychiatric disease with an unknown etiology. The research into the neurobiology of this disease led to several models aimed at explaining the link between perturbations in brain function and the manifestation of psychotic symptoms. The glutamatergic hypothesis postulates that disrupted glutamate neurotransmission may mediate cognitive and psychosocial impairments by affecting the connections between the cortex and the thalamus. In this regard, the greatest attention has been given to ionotropic NMDA receptor hypofunction. However, converging data indicates metabotropic glutamate receptors as crucial for cognitive and psychomotor function. The distribution of these receptors in the brain regions related to schizophrenia and their regulatory role in glutamate release make them promising molecular targets for novel antipsychotics. This article reviews the progress in the research on the role of metabotropic glutamate receptors in schizophrenia etiopathology. Citation: Kryszkowski, W.; Boczek, T. The G Protein-Coupled Glutamate Keywords: schizophrenia; metabotropic glutamate receptors; positive allosteric modulators; negative Receptors as Novel Molecular Targets allosteric modulators; drug development; animal models of schizophrenia; clinical trials in Schizophrenia Treatment—A Narrative Review. J. Clin. Med. 2021, 10, 1475. https://doi.org/10.3390/ jcm10071475 1. Introduction Academic Editors: Andreas Reif, Schizophrenia is a common debilitating disease affecting about 0.3–1% of the human Blazej Misiak and Jerzy Samochowiec population worldwide [1]. -

Active Filers - Metropolitan Transportation Authority 10/28/2020
Active Filers - Metropolitan Transportation Authority 10/28/2020 Name Yrs in Role Aarons, Marcel 5.42 Abayeva, Bela 5.1 Abbas, Syed 3.17 Abdallah, Ahmad 2.38 Abdallah, Thomas 8.39 Abdelkader, Ashraf 0.43 Abdelrahman, Aliaa 6.79 Abili, John 9.48 Aboagye, Nana 3.18 Abraham, George 4.64 Abraham, Sajan 9.94 Abraham-David, Ruby 1.16 Abramchayeva, Liliya 2.09 Acampora, Christopher 3.04 Accardo, Matthew 2.5 Accettura, Stephen 1.69 Accousti, James 2.07 Acerra, Paul 8.41 Acevedo, Kenneth 8.39 Acevedo, Victor 1.08 Acevedo, Wilma 2.87 Ackerman, Neil 5.21 Acosta, Leudy 0.07 Acosta, Raul 3.04 Adam, Mohamed 2.11 Adams, Benjamin 2.15 Adams, Chantay 2.23 Adams, Diane 3.84 Adams, Eric 1.58 Adams, Gerald 3.6 Adams, Julie 3.39 Addoquaye, Anna 10.88 Adeleke, Juliet 6.29 Adeleye, Ireoluwa 0.93 Adetchessi, Raoufou 0.98 Adewolu, Olasupo 3.4 Adinolfi, Nina 3.33 Adlam, Christopher 0.96 Adomah, Chereh 8.39 Aebly, Richard 5.88 Afify, Ebtesam 5.14 Afko, Koffi 4.31 Agard, Aldale 4.75 Aglitsky, Yekaterina 5.99 Agosto, Casildo 1.25 Agrawal, Sonika 2.35 Agrelo, Joshua 2.83 Agyei, Anita 6.04 Ahamed, Ali 3.76 Ahearn, Mary 3.45 Ahmed, Azad 2.42 Ahmed, Ekhlas 9.91 Ahmed, Humayun 7.19 Ahmed, Khondker 6.04 Ahmed, Margaret 4.09 Ahmed, Shahzad 1.38 Aiello, Alexandra 2.03 Aiello, Gavin 5.11 Aievoli, Steven 6.16 Aiyer, Lata 0.79 Akbar, Reinhard 4.37 Akeredolu, Elias 5.31 Akpinar, Ali 4.09 Akselrod, Leonard 2.63 Alagna, Joseph 8.04 Albano, Albert 2.26 Albano, Steven 9.91 Albert, Alyson 2.11 Albert, Andrew 1.36 Albino, Jesus 3.32 Albino, Wayne 7.97 Alcantara, Minosca 5.55 Alcide, -

Whittle-Neuropharm-2013.Pdf
Neuropharmacology 64 (2013) 414e423 Contents lists available at SciVerse ScienceDirect Neuropharmacology journal homepage: www.elsevier.com/locate/neuropharm Deep brain stimulation, histone deacetylase inhibitors and glutamatergic drugs rescue resistance to fear extinction in a genetic mouse model Nigel Whittle a,*, Claudia Schmuckermair a, Ozge Gunduz Cinar b,d, Markus Hauschild a, Francesco Ferraguti c, Andrew Holmes b,d, Nicolas Singewald a a Department of Pharmacology and Toxicology, Institute of Pharmacy and Center for Molecular Biosciences Innsbruck (CMBI), University of Innsbruck, Innrain 80 e 82/III, A-6020 Innsbruck, Austria b Laboratory of Behavioral and Genomic Neuroscience, National Institute on Alcoholism and Alcohol Abuse, National Institutes of Health, Bethesda, MD 20852, USA Center for Neuroscience and Regenerative Medicine at the Uniformed Services University of the Health Sciences, Bethesda, MD c Department of Pharmacology, Innsbruck Medical University, A-6020 Innsbruck, Austria d Center for Neuroscience and Regenerative Medicine at the Uniformed Services University of the Health Sciences, Bethesda, MD, USA article info abstract Article history: Anxiety disorders are characterized by persistent, excessive fear. Therapeutic interventions that reverse Received 30 March 2012 deficits in fear extinction represent a tractable approach to treating these disorders. We previously re- Received in revised form ported that 129S1/SvImJ (S1) mice show no extinction learning following normal fear conditioning. We 31 May 2012 now demonstrate that weak fear conditioning does permit fear reduction during massed extinction Accepted 6 June 2012 training in S1 mice, but reveals specificdeficiency in extinction memory consolidation/retrieval. Rescue of this impaired extinction consolidation/retrieval was achieved with D-cycloserine (N-methly-D-aspar- Keywords: tate partial agonist) or MS-275 (histone deacetylase (HDAC) inhibitor), applied after extinction training. -

Data Report for Fiscal Year 2020 (Highly Compensated Report)
MTA - Data Report for Fiscal Year 2020 (Highly Compensated Report) *Last Name *First Name Middle *Title *Group School Name Highest Degree Prior Work Experience Initial O'Brien James J Mgr. Maint. Contract Admin. Managerial UNKNOWN UNKNOWN MTA Agency Berani Alban Supervising Engr Electrical Managerial CUNY City College Master of Engineering Self Employed Moravec Eva M Assistant General Counsel Professional Pace University White Plains Juris Doctor Dept. of Finance OATH Angel Nichola O AVPCenBusDisTolUnit Managerial NYU Stern School of Business Master of Mechanical Engi MTA Agency Khuu Howard N Assistant Controller Managerial Baruch College Master of Business Admin Home Box Office Reis Sergio Director Ops. Tolls & Fac. Sys Managerial Long Island University Bachelor of Science Tag Americas LLC Jacobs Daniel M Sr Dir Plan Inno&Pol Ana Managerial Rutgers University Master of Engineering MTA Agency Wilkins Alphonso Senior Safety Engineer Professional High School Diploma EnviroMed Services Inc. Walker Kellie Labor Counsel Professional Boston University Law Juris Doctor NYC Department of Education Mondal Mohammad S Supervising Engineer Structure Managerial Foreign - Non US College/Unive Bachelor Civil Engineerin Department of Buildings Friman Paul Exec Asst General Counsel Professional New York University Juris Doctor NYS Supreme Court NY Prasad Indira G Sr Project Manager TSMS Professional Stevens Institute of Technolog Master of Science Mitsui O.S.K. NY Li Bin Supervising Engineer Structure Managerial Florida International Univ Doctor of Philosophy -

Andrew Rosen the Architecture of the Nervous System: • Central Nervous
Andrew Rosen The Architecture of the Nervous System: Central Nervous System (CNS) – Includes the brain and spinal cord Peripheral Nervous System (PNS) – All nerves elsewhere and are connected to the CNS via the spinal cord o Composed of the Somatic Nervous System (SNS), which has the efferent nerves that control the skeletal muscles and afferent nerves that carry information from the sense organs to CNS o Also composed of the Autonomous Nervous System (ANS), which has the efferent nerves that regulate the glands and smooth muscles of internal organs and vessels as well as afferent nerves that bring the CNS information about the internal systems . Divided into the sympathetic branch “Revs” body up for an action . Also divided into parasympathetic branch Restores the body’s internal activities to normal after an action Brain is in cerebrospinal fluid that acts as a shock absorber Anatomy of the Brain: Spinal cord that goes into brain forms the brain stem Medulla is at the bottom of the brain stem o Controls breathing, blood circulation, and maintains balance Pons is above the medulla o Controls attentiveness and governs sleep/dreaming Behind the brain stem is the cerebellum o Controls balance, coordination, and spatial reasoning The midbrain and thalamus are on top of the pons o Relay information to the forebrains o Midbrain regulates experience of pain and moods The forebrain is on top of all of these o Outer part of the forebrain is the cerebral cortex . High surface area . Deepest groove is the longitudinal fissure that splits the left cerebral hemisphere from the right . -
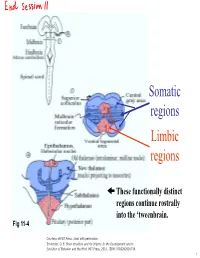
Lecture 12 Notes
Somatic regions Limbic regions These functionally distinct regions continue rostrally into the ‘tweenbrain. Fig 11-4 Courtesy of MIT Press. Used with permission. Schneider, G. E. Brain structure and its Origins: In the Development and in Evolution of Behavior and the Mind. MIT Press, 2014. ISBN: 9780262026734. 1 Chapter 11, questions about the somatic regions: 4) There are motor neurons located in the midbrain. What movements do those motor neurons control? (These direct outputs of the midbrain are not a subject of much discussion in the chapter.) 5) At the base of the midbrain (ventral side) one finds a fiber bundle that shows great differences in relative size in different species. Give examples. What are the fibers called and where do they originate? 8) A decussating group of axons called the brachium conjunctivum also varies greatly in size in different species. It is largest in species with the largest neocortex but does not come from the neocortex. From which structure does it come? Where does it terminate? (Try to guess before you look it up.) 2 Motor neurons of the midbrain that control somatic muscles: the oculomotor nuclei of cranial nerves III and IV. At this level, the oculomotor nucleus of nerve III is present. Fibers from retina to Superior Colliculus Brachium of Inferior Colliculus (auditory pathway to thalamus, also to SC) Oculomotor nucleus Spinothalamic tract (somatosensory; some fibers terminate in SC) Medial lemniscus Cerebral peduncle: contains Red corticospinal + corticopontine fibers, + cortex to hindbrain fibers nucleus (n. ruber) Tectospinal tract Rubrospinal tract Courtesy of MIT Press. Used with permission. Schneider, G. -
White Matter Tracts - Brain A143 (1)
WHITE MATTER TRACTS - BRAIN A143 (1) White Matter Tracts Last updated: August 8, 2020 CORTICOSPINAL TRACT .......................................................................................................................... 1 ANATOMY .............................................................................................................................................. 1 FUNCTION ............................................................................................................................................. 1 UNCINATE FASCICULUS ........................................................................................................................... 1 ANATOMY .............................................................................................................................................. 1 DTI PROTOCOL ...................................................................................................................................... 4 FUNCTION .............................................................................................................................................. 4 DEVELOPMENT ....................................................................................................................................... 4 CLINICAL SIGNIFICANCE ........................................................................................................................ 4 ARTICLES ..............................................................................................................................................