Journal of Thoracic Oncology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Decreased Serum Ceruloplasmin Levels Characteristically Aggravate Nigral Iron Deposition in Parkinson’S Disease Downloaded From
doi:10.1093/brain/awq319 Brain 2011: 134; 50–58 | 50 BRAIN A JOURNAL OF NEUROLOGY Decreased serum ceruloplasmin levels characteristically aggravate nigral iron deposition in Parkinson’s disease Downloaded from Lirong Jin,1,* Jian Wang,2,* Lei Zhao,1 Hang Jin,2 Guoqiang Fei,1 Yuwen Zhang,1 Mengsu Zeng2 and Chunjiu Zhong1,3,4 brain.oxfordjournals.org 1 Department of Neurology, Zhongshan Hospital and Shanghai Medical College, Fudan University, Shanghai, China 2 Department of Radiology, Zhongshan Hospital and Shanghai Medical College, Fudan University, Shanghia, China 3 State Key Laboratory of Medical Neurobiology, Shanghai Medical College, Fudan University, Shanghai, China 4 Institutes of Brain Science, Fudan University, Shanghai, China *These authors contributed equally to this work. Correspondence to: Chunjiu Zhong, MD, PhD, at Main Library L610 Lawrence Livermore Nat Lab on January 23, 2011 Department of Neurology, Zhongshan Hospital and Shanghai Medical College, Fudan University, Shanghai, China E-mail: [email protected] Correspondence may also be addressed to: Mengsu Zeng, MD, PhD, E-mail: [email protected] In vivo and post-mortem studies have demonstrated that increased nigral iron content in patients with Parkinson’s disease is a prominent pathophysiological feature. However, the mechanism and risk factors associated with nigral iron deposition in pa- tients with Parkinson’s disease have not been identified and represent a key challenge in understanding its pathogenesis and for its diagnosis. In this study, we assessed iron levels in patients with Parkinson’s disease and in age- and gender-matched control subjects by measuring phase values using magnetic resonance based susceptibility-weighted phase imaging in a 3T magnetic resonance system. -

UKI NETS 11Th National Conference 25 November 2013 the Royal College of Physicians London, UK
UKI NETS 11th National Conference 25 November 2013 The Royal College of Physicians London, UK Abstract Marking Panel Alan Anthoney (Leeds, UK) Simon Aylwin (London, UK) Dr Tu Vinh Luong (London, UK) Prakesh Manoharan (Manchester, UK) Nick Reed (Glasgow, UK) UKI NETS would like to thank their sponsors for their kind generosity: Educational Sponsors: Meeting Sponsor: UKI NETS Euro House 22 Apex Court Tel: +44-(0)1454 642277 Woodlands Fax: +44-(0)1454 642222 Bradley Stoke Email: [email protected] Bristol, BS32 4JT, UK Website: www.ukinets.org 2 Contents Pages Programme 4 – 6 Speaker Biographies 7 - 10 Abstracts 12 – 67 3 Programme CPD UKI NETS 11TH NATIONAL CONFERENCE has been approved by the Federation of the Royal Colleges of Physicians of the United Kingdom for 6 category 1 (external) CPD credits. 08:30 Registration, Coffee and Poster viewing 09:25 Welcome and opening remarks Nick Reed (Glasgow, UK) 09:30 – 11:00 Session 1 – Pancreatic NETS –who when and how to intervene? Chair: Graeme Poston 09:30 Imaging assessment of the incidental pancreatic mass Dylan Lewis (London, UK) 09:50 Management of pancreatic primary in presence of metastatic disease Massimo Falconi (Torrette-Ancona, Italy) 10:10 Localisation of insulinoma Karim Meeran (London, UK) 10:30 Surgical approach to the pancreas and parathyroid in patients with MEN1/VHL Barney Harrison (Sheffield, UK) 10:50 Open Floor Q&A 11:00 Coffee, poster viewing and exhibition 11:30 - 12:15 Session 2a – Management dilemmas - Debate – Chemotherapy is first line treatment for pancreatic metastatic -

EDDIE MARTINEZ Born: 1977, Groton Naval Base, Groton, CT Lives And
EDDIE MARTINEZ Born: 1977, Groton Naval Base, Groton, CT Lives and works in Brooklyn, NY SELECTED SOLO EXHIBITIONS 2021 Green Thumb, Blum & Poe, Los Angeles, CA Inside Thoughts, Mitchell-Innes & Nash, New York, NY New Paintings 2, Loyal Gallery, Stockholm 2019 Eddie Martinez: Open Feast, Yuz Museum, Shanghai, China Eddie Martinez: Fast Eddie, Museum of Contemporary Art, Detroit, MI EMHK19, Perrotin, Hong Kong, China 2018 White Outs, The Bronx Museum, New York, NY Blockhead Stacks, Perrotin, Hong Kong, China Love Letters and Yard Work, Mitchell-Innes & Nash, New York, NY 2017 Studio Wall, The Drawing Center, New York, NY Ants at a Picknic, Davis Museum at Wellesley College, Wellesley, MA Cowboy Town, Timothy Taylor, London 2016 Stop the world and let me off, Sorry We’re Closed, Brussels Salmon Eye, Mitchell-Innes & Nash, New York, NY 2014 Island I, Timothy Taylor Gallery, London Nomader, Kohn Gallery, Los Angeles, CA Neanderthal Jeans, Half Gallery, New York, NY 2013 Beginner Mind, Bill Brady KC, Kansas City, MO Matador, The Journal Gallery, New York, NY 2012 Studio Drawings, Half Gallery, New York, NY 2011 Seeker, Peres Projects, Berlin New York Electric, Patricia Low Contemporary, Geneva Eddie Martinez – Drawings, 2005-2011, Schwarz Contemporary, Berlin 2010 The Feast, presented by ZieherSmith, Art Positions, Art Basel Miami Beach, FL Buffet State Of Mind, Sorry We're Closed, Brussels Eddie Martinez, ZieherSmith, New York, NY 2009 News and Updates, Galerie Mikael Andersen, Copenhagen Eddie Martinez, Seomi & Tuus, Seoul 2008 Some Drawings, -

Infanticide in Early Modem Gennany: the Experience of Augsburg, Memmingen, Ulm, and Niirdlingen, 1500-1800
Infanticide in Early Modem Gennany: the experience of Augsburg, Memmingen, Ulm, and Niirdlingen, 1500-1800 Margaret Brannan Lewis Charlottesville, Virginia M.A., History, University of Virginia, 2008 B.A., History and Gennan, Furman University, 2006 A Dissertation presented to the Graduate Faculty of the University of Virginia in Candidacy for the Degree of Doctor of Philosophy Department of History University of Virginia May, 2012 i Abstract Between 1500 and 1800, over 100 women and men were arrested for infanticide or abortion in the city of Augsburg in southern Germany. At least 100 more were arrested for the same crime in the three smaller cities of Ulm, Memmingen, and Nördlingen. Faced with harsh punishments as well as social stigma if found pregnant out of wedlock, many women in early modern Europe often saw abortion or infanticide as their only option. At the same time, town councils in these southern German cities increasingly considered it their responsibility to stop this threat to the godly community and to prosecute cases of infanticide or abortion and to punish (with death) those responsible. The story of young, unmarried serving maids committing infanticide to hide their shame is well-known, but does not fully encompass the entirety of how infanticide was perceived in the early modern world. This work argues that these cases must be understood in a larger cultural context in which violence toward children was a prevalent anxiety, apparent in popular printed literature and educated legal, medical, and religious discourse alike. In the sixteenth and seventeenth centuries, this anxiety was expressed in and reinforced by woodcuts featuring mass murders of families, deformed babies, and cannibalism of infants by witches and other dark creatures. -

ACFEA Newsletter 97/98
To ur N o t e s PERFORMING ARTS NEWS FROM AROUND THE WORLD 1997-1998 Highlights Trinity Church Traces its Roots Duquesne Wins at Marktoberdorf Alaska Children in Europe Rutgers Women Explore Ireland California Youth Orchestra in Britain and Ireland Spreading Goodwill in the Baltic Republics Missa Gaia on the Riviera Honor Band at WASBE Portland Girlchoir in Australia Festival Sir Simon Rattle conducts The Damanation of Faust in Birmingham Opportunities in the Pacific Northwest ‘VOICES IN For nine days in July, Morrell in the Sheldonian Theatre, Ox- THE CITY’ A the City of Birming- ford. To celebrate the 100th birthday of Alabama Choir Does Europe! ham (England) rang the recording company EMI, the Cleve- HUGE to the sounds of land Orchestra Chorus then joined forces Amherst in Spain and Portugal SUCCESS superlative choral with the City of Birmingham Symphony singing. More than Orchestra and Chorus to give a stunning 2,000 singers from Great Britain, the USA performance of Walton’s Belshazzar’s and Canada took part in over 20 choral Feast, conducted by Sir Simon Rattle in concerts, workshops and master classes, Symphony Hall, Birmingham. They then thrilling the large audiences which filled recorded the work for release by EMI later Symphony Hall and other venues. this year. It was the first major choral festival The final concert of ‘Voices in the in Birmingham since the great triennial City’ proved to be another blockbuster, festivals of the 19th century, which saw with over 300 singers sharing the plat- the first performances of Mendelssohn’s form with a much enlarged CBSO to Elijah and Elgar’s The Dream of perform Berlioz’s dramatic oratorio, The Gerontius, among others. -

Trustees' Annual Report and Financial Statements 31 March 2016
THE FRANCIS CRICK INSTITUTE LIMITED A COMPANY LIMITED BY SHARES TRUSTEES’ ANNUAL REPORT AND FINANCIAL STATEMENTS 31 MARCH 2016 Charity registration number: 1140062 Company registration number: 6885462 The Francis Crick Institute Accounts 2016 CONTENTS INSIDE THIS REPORT Trustees’ report (incorporating the Strategic report and Directors’ report) 1 Independent auditor’s report 12 Consolidated statement of financial activities 13 Balance sheets 14 Cash flow statements 15 Notes to the financial statements 16 1 TRUSTEES’ REPORT (INCORPORATING THE STRATEGIC REPORT AND DIRECTORS’ REPORT) The trustees present their annual directors’ report together with the consolidated financial statements for the charity and its subsidiary (together, ‘the Group’) for the year ended 31 March 2016, which are prepared to meet the requirements for a directors’ report and financial statements for Companies Act purposes. The financial statements comply with the Charities Act 2011, the Companies Act 2006, and the Statement of Recommended Practice applicable to charities preparing their accounts in accordance with the Financial Reporting Standard applicable in the UK (FRS102) effective 1 January 2015 (Charity SORP). The trustees’ report includes the additional content required of larger charities. REFERENCE AND ADMINISTRATIVE DETAILS The Francis Crick Institute Limited (‘the charity’, ‘the Institute’ or ‘the Crick) is registered with the Charity Commission, charity number 1140062. The charity has operated and continues to operate under the name of the Francis Crick -

Paterson Institute for Cancer Research Scientific Report 2008 Contents
paterson institute for cancer research scientific report 2008 cover images: Main image supplied by Karim Labib and Alberto sanchez-Diaz (cell cycle Group). Budding yeast cells lacking the inn1 protein are unable to complete cytokinesis. these cells express a fusion of a green fluorescent protein to a marker of the plasma membrane, and have red fluorescent proteins attached to components of the spindle poles and actomyosin ring (sanchez-Diaz et al., nature cell Biology 2008; 10: 395). Additional images: front cover image supplied by Helen rushton, simon Woodcock and Angeliki Malliri (cell signalling Group). the image is of a mitotic spindle in fixed MDcK (Madin-Darby canine kidney) epithelial cells, which have been stained with an anti-beta tubulin antibody (green), DApi (blue) and an anti-centromere antibody (crest, red) which recognises the kinetochores of the chromosomes. the image was taken on the spinning disk confocal microscope using a 150 x lens. rear cover image supplied by Andrei ivanov and tim illidge (targeted therapy Group). Visualisation of tubulin (green) and quadripolar mitosis (DnA stained with DApi), Burkitt’s lymphoma namalwa cell after 10 Gy irradiation. issn 1740-4525 copyright 2008 © cancer research UK Paterson Institute for Cancer Research Scientific Report 2008 Contents 4 Director’s Introduction Researchers’ pages – Paterson Institute for Cancer Research 8 Crispin Miller Applied Computational Biology and Bioinformatics 10 Geoff Margison Carcinogenesis 12 Karim Labib Cell Cycle 14 Iain Hagan Cell Division 16 Nic Jones -

World Scientists' Warning of a Climate Emergency
Supplemental File S1 for the article “World Scientists’ Warning of a Climate Emergency” published in BioScience by William J. Ripple, Christopher Wolf, Thomas M. Newsome, Phoebe Barnard, and William R. Moomaw. Contents: List of countries with scientist signatories (page 1); List of scientist signatories (pages 1-319). List of 153 countries with scientist signatories: Albania; Algeria; American Samoa; Andorra; Argentina; Australia; Austria; Bahamas (the); Bangladesh; Barbados; Belarus; Belgium; Belize; Benin; Bolivia (Plurinational State of); Botswana; Brazil; Brunei Darussalam; Bulgaria; Burkina Faso; Cambodia; Cameroon; Canada; Cayman Islands (the); Chad; Chile; China; Colombia; Congo (the Democratic Republic of the); Congo (the); Costa Rica; Côte d’Ivoire; Croatia; Cuba; Curaçao; Cyprus; Czech Republic (the); Denmark; Dominican Republic (the); Ecuador; Egypt; El Salvador; Estonia; Ethiopia; Faroe Islands (the); Fiji; Finland; France; French Guiana; French Polynesia; Georgia; Germany; Ghana; Greece; Guam; Guatemala; Guyana; Honduras; Hong Kong; Hungary; Iceland; India; Indonesia; Iran (Islamic Republic of); Iraq; Ireland; Israel; Italy; Jamaica; Japan; Jersey; Kazakhstan; Kenya; Kiribati; Korea (the Republic of); Lao People’s Democratic Republic (the); Latvia; Lebanon; Lesotho; Liberia; Liechtenstein; Lithuania; Luxembourg; Macedonia, Republic of (the former Yugoslavia); Madagascar; Malawi; Malaysia; Mali; Malta; Martinique; Mauritius; Mexico; Micronesia (Federated States of); Moldova (the Republic of); Morocco; Mozambique; Namibia; Nepal; -

The Ginger Fox's Two Crowns Central Administration and Government in Sigismund of Luxembourg's Realms
Doctoral Dissertation THE GINGER FOX’S TWO CROWNS CENTRAL ADMINISTRATION AND GOVERNMENT IN SIGISMUND OF LUXEMBOURG’S REALMS 1410–1419 By Márta Kondor Supervisor: Katalin Szende Submitted to the Medieval Studies Department, Central European University, Budapest in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Medieval Studies, CEU eTD Collection Budapest 2017 Table of Contents I. INTRODUCTION 6 I.1. Sigismund and His First Crowns in a Historical Perspective 6 I.1.1. Historiography and Present State of Research 6 I.1.2. Research Questions and Methodology 13 I.2. The Luxembourg Lion and its Share in Late-Medieval Europe (A Historical Introduction) 16 I.2.1. The Luxembourg Dynasty and East-Central-Europe 16 I.2.2. Sigismund’s Election as King of the Romans in 1410/1411 21 II. THE PERSONAL UNION IN CHARTERS 28 II.1. One King – One Land: Chancery Practice in the Kingdom of Hungary 28 II.2. Wearing Two Crowns: the First Years (1411–1414) 33 II.2.1. New Phenomena in the Hungarian Chancery Practice after 1411 33 II.2.1.1. Rex Romanorum: New Title, New Seal 33 II.2.1.2. Imperial Issues – Non-Imperial Chanceries 42 II.2.2. Beginnings of Sigismund’s Imperial Chancery 46 III. THE ADMINISTRATION: MOBILE AND RESIDENT 59 III.1. The Actors 62 III.1.1. At the Travelling King’s Court 62 III.1.1.1. High Dignitaries at the Travelling Court 63 III.1.1.1.1. Hungarian Notables 63 III.1.1.1.2. Imperial Court Dignitaries and the Imperial Elite 68 III.1.1.2. -

Small Arms in the Hands of Children
Christopher Steinmetz (BITS) German Arms Exports and Child Soldiers Small Arms in the Hands of Children Cooperation partner Editor German Coalition to Stop the Use of Child Soldiers Help for Children in Need Christopher Steinmetz (BITS) German Arms Exports and Child Soldiers Small Arms in the Hands of Children 2 Imprint Author Christopher Steinmetz, research associate, Berlin Information-center for Transatlantic security – BITS Cooperation Otfried Nassauer (BITS) Coordination and Editor Ralf Willinger/terre des hommes Design kippconcept gmbh, Bonn Published by and on behalf Brot für die Welt Kindernothilfe e.V. terre des hommes e.V. World Vision Deutschland e.V Supported by Deutsches Komitee für UNICEF e.V. Deutsche Friedensgesellschaft – DFG-VK e.V. Pax Christi – Deutsche Sektion Ohne Rüstung Leben e.V. Photos The publishing organisations Kindernothilfe and Cover photo: Child soldier with German G3-rifle, terre des hommes are members of the German Coalition photographer unknown to Stop the Use of Child Soldiers. page 9: Sebastian Bolesch www.kindersoldaten.info page 11: Guillaume Briquet / AFP / GettyImages page 13: Hans-Martin Grosse-Oetringhaus / terre des hommes The publishing organisations Brot für die Welt and page 26 – 27: Jacob_Wire / dpa_Picture Alliance terre des hommes are members of the coalition page 31: Hans-Martin Grosse-Oetringhaus / terre des hommes „Aktion Aufschrei – Stoppt den Waffenhandel“. page 48: David Longstreath / AP Photo / dpa_Picture Alliance www.aufschrei-waffenhandel.de page 55: Jacob_Wire / dpa_Picture Alliance page 64: Sebastian Bolesch The views expressed in this publication are those page 69: William Martínez / Fundación Dos Mundos of the author, not necessarily those of the supporting organizations. -

2017 MAJOR EURO Music Festival CALENDAR Sziget Festival / MTI Via AP Balazs Mohai
2017 MAJOR EURO Music Festival CALENDAR Sziget Festival / MTI via AP Balazs Mohai Sziget Festival March 26-April 2 Horizon Festival Arinsal, Andorra Web www.horizonfestival.net Artists Floating Points, Motor City Drum Ensemble, Ben UFO, Oneman, Kink, Mala, AJ Tracey, Midland, Craig Charles, Romare, Mumdance, Yussef Kamaal, OM Unit, Riot Jazz, Icicle, Jasper James, Josey Rebelle, Dan Shake, Avalon Emerson, Rockwell, Channel One, Hybrid Minds, Jam Baxter, Technimatic, Cooly G, Courtesy, Eva Lazarus, Marc Pinol, DJ Fra, Guim Lebowski, Scott Garcia, OR:LA, EL-B, Moony, Wayward, Nick Nikolov, Jamie Rodigan, Bahia Haze, Emerald, Sammy B-Side, Etch, Visionobi, Kristy Harper, Joe Raygun, Itoa, Paul Roca, Sekev, Egres, Ghostchant, Boyson, Hampton, Jess Farley, G-Ha, Pixel82, Night Swimmers, Forbes, Charline, Scar Duggy, Mold Me With Joy, Eric Small, Christer Anderson, Carina Helen, Exswitch, Seamus, Bulu, Ikarus, Rodri Pan, Frnch, DB, Bigman Japan, Crawford, Dephex, 1Thirty, Denzel, Sticky Bandit, Kinno, Tenbagg, My Mate From College, Mr Miyagi, SLB Solden, Austria June 9-July 10 DJ Snare, Ambiont, DLR, Doc Scott, Bailey, Doree, Shifty, Dorian, Skore, March 27-April 2 Web www.electric-mountain-festival.com Jazz Fest Vienna Dossa & Locuzzed, Eksman, Emperor, Artists Nervo, Quintino, Michael Feiner, Full Metal Mountain EMX, Elize, Ernestor, Wastenoize, Etherwood, Askery, Rudy & Shany, AfroJack, Bassjackers, Vienna, Austria Hemagor, Austria F4TR4XX, Rapture,Fava, Fred V & Grafix, Ostblockschlampen, Rafitez Web www.jazzfest.wien Frederic Robinson, -
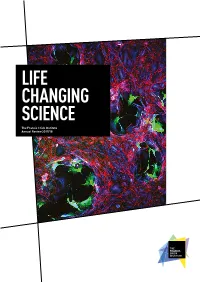
Life Changing Science
LIFE CHANGING SCIENCE The Francis Crick Institute Annual Review 2017/18 AN INSTITUTE FOR DISCOVERY Our commitment to excellence, our emphasis on multidisciplinary research, our focus on young and emerging talent and our novel ways of partnership working are some of the factors that set the Crick apart. Front cover Vaccinia virus infection (green) disrupts a layer of epithelial cells (red/blue). Courtesy of Michael Way, Group Leader at the Crick. INTRODUCTION 2 Who we are Our year at a glance 2 Introduction by Paul Nurse 4 The Francis Crick Institute is a biomedical Progress against our strategy 6 discovery institute dedicated to understanding the RESEARCH HIGHLIGHTS 10 Cancer-causing mutation fundamental biology underlying health and disease. suppresses immune system 11 Our work is helping to build an understanding of Predicting lung cancer’s return 12 New understanding of human why disease develops and to translate discoveries embryo development 14 Chemical attraction could improve into new ways to prevent, diagnose and treat cancer immunotherapy 16 illnesses such as cancer, heart disease, stroke, Genes linked to malaria parasites’ persistence 17 infections and neurodegenerative diseases. Architecture of our ‘second brain’ 18 Cause of infertility side-stepped in mice 19 Mechanism for spinal cord development discovered 20 A new layer of complexity in embryo development 21 Two DNAs wedded with this ring 22 Unravelling how DNA gets copied 23 Telomerase’s dark side discovered 24 REVIEW OF THE YEAR 26 New group leaders arrive 27 Joined-up thinking 30 Focusing on the molecules of life 32 CryoEM at the Crick 34 Bringing academia and industry closer together 36 The people making research happen 38 Patterns in art and science 40 Rewarding research 42 Appointments 43 Supporting new discoveries 44 Our vision What’s inside Our vision is to be a world- We bring together outstanding scientists Science feature 32 leading multidisciplinary from all disciplines and carry out research Sophisticated microscopy is being biomedical research institute.