Precise Colocalization of Interacting Structural and Pigmentary Elements Generates Extensive Color Pattern Variation in Phelsuma Lizards Saenko Et Al
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dynamically Tunable Plasmonic Structural Color
University of Central Florida STARS Electronic Theses and Dissertations, 2004-2019 2018 Dynamically Tunable Plasmonic Structural Color Daniel Franklin University of Central Florida Part of the Physics Commons Find similar works at: https://stars.library.ucf.edu/etd University of Central Florida Libraries http://library.ucf.edu This Doctoral Dissertation (Open Access) is brought to you for free and open access by STARS. It has been accepted for inclusion in Electronic Theses and Dissertations, 2004-2019 by an authorized administrator of STARS. For more information, please contact [email protected]. STARS Citation Franklin, Daniel, "Dynamically Tunable Plasmonic Structural Color" (2018). Electronic Theses and Dissertations, 2004-2019. 5880. https://stars.library.ucf.edu/etd/5880 DYNAMICALLY TUNABLE PLASMONIC STRUCTURAL COLOR by DANIEL FRANKLIN B.S. Missouri University of Science and Technology, 2011 A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Department of Physics in the College of Sciences at the University of Central Florida Orlando, Florida Spring Term 2018 Major Professor: Debashis Chanda © 2018 Daniel Franklin ii ABSTRACT Functional surfaces which can control light across the electromagnetic spectrum are highly desirable. With the aid of advanced modeling and fabrication techniques, researchers have demonstrated surfaces with near arbitrary tailoring of reflected/transmitted amplitude, phase and polarization - the applications for which are diverse as light itself. These systems often comprise of structured metals and dielectrics that, when combined, manifest resonances dependent on structural dimensions. This attribute provides a convenient and direct path to arbitrarily engineer the surface’s optical characteristics across many electromagnetic regimes. -

The Case of Deirocheline Turtles
bioRxiv preprint doi: https://doi.org/10.1101/556670; this version posted February 21, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Body coloration and mechanisms of colour production in Archelosauria: 2 The case of deirocheline turtles 3 Jindřich Brejcha1,2*†, José Vicente Bataller3, Zuzana Bosáková4, Jan Geryk5, 4 Martina Havlíková4, Karel Kleisner1, Petr Maršík6, Enrique Font7 5 1 Department of Philosophy and History of Science, Faculty of Science, Charles University, Viničná 7, Prague 6 2, 128 00, Czech Republic 7 2 Department of Zoology, Natural History Museum, National Museum, Václavské nám. 68, Prague 1, 110 00, 8 Czech Republic 9 3 Centro de Conservación de Especies Dulceacuícolas de la Comunidad Valenciana. VAERSA-Generalitat 10 Valenciana, El Palmar, València, 46012, Spain. 11 4 Department of Analytical Chemistry, Faculty of Science, Charles University, Hlavova 8, Prague 2, 128 43, 12 Czech Republic 13 5 Department of Biology and Medical Genetics, 2nd Faculty of Medicine, Charles University and University 14 Hospital Motol, V Úvalu 84, 150 06 Prague, Czech Republic 15 6 Department of Food Science, Faculty of Agrobiology, Food, and Natural Resources, Czech University of Life 16 Sciences, Kamýcká 129, Prague 6, 165 00, Czech Republic 17 7 Ethology Lab, Cavanilles Institute of Biodiversity and Evolutionary Biology, University of Valencia, C/ 18 Catedrátic José Beltrán Martinez 2, Paterna, València, 46980, Spain 19 Keywords: Chelonia, Trachemys scripta, Pseudemys concinna, nanostructure, pigments, chromatophores 20 21 Abstract 22 Animal body coloration is a complex trait resulting from the interplay of multiple colour-producing mechanisms. -
Skin Patterning in Octopus Vulgaris and Its Importance for Camouflage
Skin patterning in Octopus vulgaris anditsimportance for camouflage DORSAL ANTERiOR POSTERIOR VENTRAL Wilma Meijer-Kuiper D490 Dqo "Scriptie"entitled: j Skin patterning in Octopus vulgaris and its importance for camouflage Author: Wilma Meijer-Kuiper. Supervisor: Dr. P.J. Geerlink. April, 1993. Cover figure from Wells, 1978. Department of Marine Biology, University of Groningen, Kerklaan 30, 9750 AA Haren, The Netherlands. TABLE OF CONTENTS PAGE Abstract 1 1. Introduction 2 1.1. Camouflage in marine invertebrates 2 1 .2. Cephalopods 2 2. General biology of the octopods 4 2.1. Octopus vulgar/s 4 3. Skin patterning in Octopus 6 3.1. Patterns 6 3.2. Components 8 3.3. Units 9 3.4. Elements 10 3.4.1. Chromatophores 10 3.4.2. Reflector cells and iridophores 16 3.4.3. Leucophores 18 4. The chromatic unit 20 5. Nervous system 22 5.1. Motor units 22 5.2. Electrical stimulation 22 5.3. Projection of nerves on the skin surface 24 5.4. The nervous control system 25 5.5. Neurotransmitters 27 6. Vision in cephalopods, particularly in O.vu!garis 28 6.1. Electro-physiological experiments 28 6.2. Photopigments and receptor cells 28 6.3. Behavioural (training) experiments 29 6.4. Eye movements and optomotor responses 29 7. Camouflage in a colour-blind animal 31 7.1. Matching methods 31 7.2. Countershading reflex 35 8. Conclusive remarks 36 Literature 38 ABSTRACT Camouflage is a method by which animals obtain concealment from other animals by blending in with their environment. Of the cephalopods, the octopods have an extra-ordinary ability to match their surroundings by changing the colour and texture of their skin. -

Comparative Visualization for Two-Dimensional Gas Chromatography
COMPARATIVE VISUALIZATION FOR TWO-DIMENSIONAL GAS CHROMATOGRAPHY by Ben Hollingsworth A THESIS Presented to the Faculty of The Graduate College at the University of Nebraska In Partial Fulfillment of Requirements For the Degree of Master of Science Major: Computer Science Under the Supervision of Professor Stephen E. Reichenbach Lincoln, Nebraska December, 2004 COMPARATIVE VISUALIZATION FOR TWO-DIMENSIONAL GAS CHROMATOGRAPHY Ben Hollingsworth, M.S. University of Nebraska, 2004 Advisor: Stephen E. Reichenbach This work investigates methods for comparing two datasets from comprehensive two-dimensional gas chromatography (GC×GC). Because GC×GC introduces incon- sistencies in feature locations and pixel magnitudes from one dataset to the next, several techniques have been developed for registering two datasets to each other and normalizing their pixel values prior to the comparison process. Several new methods of image comparison improve upon pre-existing generic methods by taking advantage of the image characteristics specific to GC×GC data. A newly developed colorization scheme for difference images increases the amount of information that can be pre- sented in the image, and a new “fuzzy difference” algorithm highlights the interesting differences between two scalar rasters while compensating for slight misalignment between features common to both images. In addition to comparison methods based on two-dimensional images, an inter- active three-dimensional viewing environment allows analysts to visualize data using multiple comparison methods simultaneously. Also, high-level features extracted from the images may be compared in a tabular format, side by side with graphical repre- sentations overlaid on the image-based comparison methods. These image processing techniques and high-level features significantly improve an analyst’s ability to detect similarities and differences between two datasets. -

Volume 2. Animals
AC20 Doc. 8.5 Annex (English only/Seulement en anglais/Únicamente en inglés) REVIEW OF SIGNIFICANT TRADE ANALYSIS OF TRADE TRENDS WITH NOTES ON THE CONSERVATION STATUS OF SELECTED SPECIES Volume 2. Animals Prepared for the CITES Animals Committee, CITES Secretariat by the United Nations Environment Programme World Conservation Monitoring Centre JANUARY 2004 AC20 Doc. 8.5 – p. 3 Prepared and produced by: UNEP World Conservation Monitoring Centre, Cambridge, UK UNEP WORLD CONSERVATION MONITORING CENTRE (UNEP-WCMC) www.unep-wcmc.org The UNEP World Conservation Monitoring Centre is the biodiversity assessment and policy implementation arm of the United Nations Environment Programme, the world’s foremost intergovernmental environmental organisation. UNEP-WCMC aims to help decision-makers recognise the value of biodiversity to people everywhere, and to apply this knowledge to all that they do. The Centre’s challenge is to transform complex data into policy-relevant information, to build tools and systems for analysis and integration, and to support the needs of nations and the international community as they engage in joint programmes of action. UNEP-WCMC provides objective, scientifically rigorous products and services that include ecosystem assessments, support for implementation of environmental agreements, regional and global biodiversity information, research on threats and impacts, and development of future scenarios for the living world. Prepared for: The CITES Secretariat, Geneva A contribution to UNEP - The United Nations Environment Programme Printed by: UNEP World Conservation Monitoring Centre 219 Huntingdon Road, Cambridge CB3 0DL, UK © Copyright: UNEP World Conservation Monitoring Centre/CITES Secretariat The contents of this report do not necessarily reflect the views or policies of UNEP or contributory organisations. -

Pigment Pattern Formation in Zebrafish 443
MICROSCOPY RESEARCH AND TECHNIQUE 58:442–455 (2002) Pigment Pattern Formation in Zebrafish: A Model for Developmental Genetics and the Evolution of Form 1 1,2 IAN K. QUIGLEY AND DAVID M. PARICHY * 1Section of Integrative Biology, University of Texas at Austin, Austin, Texas 78712 2Section of Molecular, Cell and Developmental Biology and Institute for Cellular and Molecular Biology University of Texas at Austin, Austin, Texas 78712 ABSTRACT The zebrafish Danio rerio is an emerging model organism for understanding vertebrate development and genetics. One trait of both historical and recent interest is the pattern formed by neural crest–derived pigment cells, or chromatophores, which include black melano- phores, yellow xanthophores, and iridescent iridophores. In zebrafish, an embryonic and early larval pigment pattern consists of several stripes of melanophores and iridophores, whereas xanthophores are scattered widely over the flank. During metamorphosis, however, this pattern is transformed into that of the adult, which comprises several dark stripes of melanophores and iridophores that alternate with light stripes of xanthophores and iridophores. In this review, we place zebrafish relative to other model and non-model species; we review what is known about the processes of chromatophore specification, differentiation, and morphogenesis during the develop- ment of embryonic and adult pigment patterns, and we address how future studies of zebrafish will likely aid our understanding of human disease and the evolution of form. Microsc. Res. Tech. 58: 442–455, 2002. © 2002 Wiley-Liss, Inc. INTRODUCTION number of genetic mutants have been isolated that To generate the adult body plan, cells must differen- affect pigmentation. For example, a variety of pigment tiate, proliferate, die, and migrate. -

Painted Wood: History and Conservation
PART FOUR Investigations and Treatment 278 Monochromy, Polychromy, and Authenticity The Cloisters’ Standing Bishop Attributed to Tilman Riemenschneider Michele D. Marincola and Jack Soultanian 1975, Standing Bishop was acquired for The Cloisters collection, the Metropolitan Museum of IArt, New York. This piece—considered at purchase to be a mature work of Tilman Riemenschneider (ca. 1460–1531), a leading German mas- ter of Late Gothic sculpture—was intended to complement early works by the artist already in the collection. The sculpture (Fig. 1) is indisputably in the style of Riemenschneider; furthermore, its provenance (established to before 1907) includes the renowned Munich collection of Julius Böhler.1 The Standing Bishop was accepted as an autograph work by the great Riemenschneider scholar Justus Bier (1956), who was reversing his earlier opinion. It has been compared stylistically to a number of works by Riemenschneider from about 1505–10. In the 1970s, a research project was begun by art historians and conservators in Germany to establish the chronology and authorship of a group of sculptures thought to be early works of Riemenschneider. The Cloisters’ sculptures, including the Standing Bishop, were examined as part of the project, and cross sections were sent to Munich for analysis by Hermann Kühn. This research project resulted in an exhibition of the early work of Riemenschneider in Würzburg in 1981; The Cloisters sent two sculptures from its collection, but the loan of the Standing Bishop was not requested. Certain stylistic anomalies of the figure, as well as several Figure 1 technical peculiarities discussed below, contributed to the increasing suspi- Standing Bishop, attributed to Tilman cion that it was not of the period. -

The Biology and Ecology of the Common Cuttlefish (Sepia Officinalis)
Supporting Sustainable Sepia Stocks Report 1: The biology and ecology of the common cuttlefish (Sepia officinalis) Daniel Davies Kathryn Nelson Sussex IFCA 2018 Contents Summary ................................................................................................................................................. 2 Acknowledgements ................................................................................................................................. 2 Introduction ............................................................................................................................................ 3 Biology ..................................................................................................................................................... 3 Physical description ............................................................................................................................ 3 Locomotion and respiration ................................................................................................................ 4 Vision ................................................................................................................................................... 4 Chromatophores ................................................................................................................................. 5 Colour patterns ................................................................................................................................... 5 Ink sac and funnel organ -

Flip Your Lid for Squid!
FLIP YOUR LID FOR SQUID! Adapted from http://njseagrant.org/wp-content/uploads/2014/03/squid_dissection.pdf PURPOSE Students learn the basics of squid anatomy and physiology by conducting a squid dissection. MATERIALS • frozen whole squid—not “clean” and not cut (get whole squid in frozen section of the grocery store) • hand lenses • dissection pan (plate) • scissors • tweezers • probe or toothpick • paper towels PROCEDURE Dissection of External Anatomy • Have students observe their squids for a few moments looking at the relationship between head and the rest of the body. Then ask students why they think squid are in the family of cephalopods (head-foots). • Tentacles and Arms: Have students count the number of arms. They may use the toothpicks to spread out the squid arms. Ask if all the arms look the same? There should be eight short arms and 2 long arms. Explain that the long thin arms with suckers only at the ends are called tentacles. The tentacles large ends with suckers are known as clubs. The tentacles are used to strike out and capture prey. The eight arms are used to hold onto prey when captured and bring food into its mouth. • Suction Cups: Student may use the magnifying lenses to get a closer look at the suction cups on each arm and tentacles. The suction cups have a small-toothed ring around each one and they are on short stalks. The suction cups are like suckers that help the arms hold onto the prey when it is captured and tries to escape. • Beak and Buccal Mass: Located within the circle of arms students can locate the squids mouth which is a beak. -
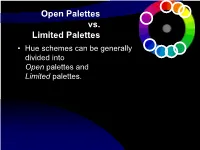
Compositional Effects of Color
Open Palettes vs. Limited Palettes • Hue schemes can be generally divided into Open palettes and Limited palettes. Open Palettes vs. Limited Palettes • Open palettes allow any hue to be present — whether naturalistic color or randomly selected hues or expressive- intuitively selected hues are used. • Limited Palettes confine the hues used to some pre- planned strategy. Structured hue schemes (e.g. analogous, complementary, triadic, etc.) are limited-hue-plans that confine colors to only a few hues based upon a structure that selects hues by their relative positions on a hue wheel. Open Palette- vs.- Limited Palette vs. Structured Palette • Limited Palette concept simply acknowledges that only a small selection of colors are used. Typically, but not always, involving a structured palette. • Structured Palette concept refers to the usual “color schemes” — that is, a “structure” of monochromatic, or of Complementary, or split complementary hue selections. The hues that are used in the palette are selected according to some scheme, plan or structure. • Open Palette is an un-structured palette. Hues may be selected from any region of the color wheel. No structure is intentionally planned or imposed. Colors are most often applied intuitively, rather than analytically. Open Palette • (p. 53) A color scheme that uses hues from all over the color wheel. • The risk: Potentially chaotic and disunified. • The potential: often rich & visually dynamic. • A strategy: When an open palette is daringly used, some other characteristics of the design must provide unity – to hold it all together. Often a simple value pattern is used. [see Matisse and the Fauves] Variety, Chaos, & Fragmentation – dissolving unity • Some designers choose to let go of any planned or structured color scheme. -

Geckos: Day Geckos, Tokay Geckos Plus New Caledonians and More!
GECKOS: DAY GECKOS, TOKAY GECKOS PLUS NEW CALEDONIANS AND MORE! Author: Julie Bergman Number of Pages: 159 pages Published Date: 01 Oct 2006 Publisher: Advanced Vivarium Systems Inc.,U.S. Publication Country: Santee, CA, United States Language: English ISBN: 9781882770892 DOWNLOAD: GECKOS: DAY GECKOS, TOKAY GECKOS PLUS NEW CALEDONIANS AND MORE! Geckos: Day Geckos, Tokay Geckos Plus New Caledonians and More! PDF Book I miss seeing them and hearing their calls. Why not leave us a comment and tell us what you think about Tokay geckos and their morphs that are being developed! View Archived Comments. A single adult leachianus can be housed in an upright screen enclosure no smaller than 18 by 18 by 24 inches tall larger would be even better , or a gallon aquarium with a screen top. Day Gecko Books. Do leopard geckos for sale stop eating when they shed? Once we start having Captive Bred Tokays to work with people will do far better keeping and breeding them and having friendly Geckos. Day geckos are great escape artists. Male mossy gecko, rare breed. Pets Reptiles Lizards Geckos Andover. Humidity is fairly important to this species. Offer both horizontal and vertical decor such as bamboo, cork bark, branches. Albino Leopard Geckos. They love crikets , worms and coconut water. Local delivery around Choose products made with sustainable ingredients, such as Smithsonian certified Bird Friendly coffees , which support farmers striving to limit their impact on wildlife and habitat. Follow on Instagram. Tokek Sabah 1. Make sure it knows that it is just your hand. Hatchlings are very flighty and will jump, but with intermittent gentle handling they will calm down. -

Color Theory
color theory What is color theory? Color Theory is a set of principles used to create harmonious color combinations. Color relationships can be visually represented with a color wheel — the color spectrum wrapped onto a circle. The color wheel is a visual representation of color theory: According to color theory, harmonious color combinations use any two colors opposite each other on the color wheel, any three colors equally spaced around the color wheel forming a triangle, or any four colors forming a rectangle (actually, two pairs of colors opposite each other). The harmonious color combinations are called color schemes – sometimes the term 'color harmonies' is also used. Color schemes remain harmonious regardless of the rotation angle. Monochromatic Color Scheme The monochromatic color scheme uses variations in lightness and saturation of a single color. This scheme looks clean and elegant. Monochromatic colors go well together, producing a soothing effect. The monochromatic scheme is very easy on the eyes, especially with blue or green hues. Analogous Color Scheme The analogous color scheme uses colors that are adjacent to each other on the color wheel. One color is used as a dominant color while others are used to enrich the scheme. The analogous scheme is similar to the monochromatic, but offers more nuances. Complementary Color Scheme The complementary color scheme consists of two colors that are opposite each other on the color wheel. This scheme looks best when you place a warm color against a cool color, for example, red versus green-blue. This scheme is intrinsically high-contrast. Split Complementary Color Scheme The split complementary scheme is a variation of the standard complementary scheme.