A Framework for Adaptive Management of Horseshoe Crab Harvest in the Delaware Bay Constrained by Red Knot Conservation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

No General Shift in Spring Migration Phenology by Eastern North American Birds Since 1970
bioRxiv preprint doi: https://doi.org/10.1101/2021.05.25.445655; this version posted May 26, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. No general shift in spring migration phenology by eastern North American birds since 1970 André Desrochers Département des sciences du bois et de la forêt, Université Laval, Québec, Canada Andra Florea Observatoire d’oiseaux de Tadoussac, Québec, Canada Pierre-Alexandre Dumas Observatoire d’oiseaux de Tadoussac, Québec, Canada, and Département des sci- ences du bois et de la forêt, Université Laval, Québec, Canada We studied the phenology of spring bird migration from eBird and ÉPOQ checklist programs South of 49°N in the province of Quebec, Canada, between 1970 and 2020. 152 species were grouped into Arctic, long-distance, and short-distance migrants. Among those species, 75 sig- nificantly changed their migration dates, after accounting for temporal variability in observation effort, species abundance, and latitude. But in contrast to most studies on the subject, we found no general advance in spring migration dates, with 36 species advancing and 39 species delaying their migration. Several early-migrant species associated to open water advanced their spring mi- gration, possibly due to decreasing early-spring ice cover in the Great Lakes and the St-Lawrence river since 1970. Arctic breeders and short-distance migrants advanced their first arrival dates more than long-distance migrants not breeding in the arctic. -

List of Shorebird Profiles
List of Shorebird Profiles Pacific Central Atlantic Species Page Flyway Flyway Flyway American Oystercatcher (Haematopus palliatus) •513 American Avocet (Recurvirostra americana) •••499 Black-bellied Plover (Pluvialis squatarola) •488 Black-necked Stilt (Himantopus mexicanus) •••501 Black Oystercatcher (Haematopus bachmani)•490 Buff-breasted Sandpiper (Tryngites subruficollis) •511 Dowitcher (Limnodromus spp.)•••485 Dunlin (Calidris alpina)•••483 Hudsonian Godwit (Limosa haemestica)••475 Killdeer (Charadrius vociferus)•••492 Long-billed Curlew (Numenius americanus) ••503 Marbled Godwit (Limosa fedoa)••505 Pacific Golden-Plover (Pluvialis fulva) •497 Red Knot (Calidris canutus rufa)••473 Ruddy Turnstone (Arenaria interpres)•••479 Sanderling (Calidris alba)•••477 Snowy Plover (Charadrius alexandrinus)••494 Spotted Sandpiper (Actitis macularia)•••507 Upland Sandpiper (Bartramia longicauda)•509 Western Sandpiper (Calidris mauri) •••481 Wilson’s Phalarope (Phalaropus tricolor) ••515 All illustrations in these profiles are copyrighted © George C. West, and used with permission. To view his work go to http://www.birchwoodstudio.com. S H O R E B I R D S M 472 I Explore the World with Shorebirds! S A T R ER G S RO CHOOLS P Red Knot (Calidris canutus) Description The Red Knot is a chunky, medium sized shorebird that measures about 10 inches from bill to tail. When in its breeding plumage, the edges of its head and the underside of its neck and belly are orangish. The bird’s upper body is streaked a dark brown. It has a brownish gray tail and yellow green legs and feet. In the winter, the Red Knot carries a plain, grayish plumage that has very few distinctive features. Call Its call is a low, two-note whistle that sometimes includes a churring “knot” sound that is what inspired its name. -

Status of the Red Knot (Calidris Canutus Rufa) in the Western Hemisphere
Status of the Red Knot ( STATUS OF THE RED KNOT (CALIDRIS CANUTUS RUFA) IN THE WESTERN HEMISPHERE Calidris canutus rufa LAWRENCE J. NILES, HUMPHREY P. SITTERS, AMANDA D. DEY, PHILIP W. ATKINSON, ALLAN J. BAKER, KAREN A. BENNETT, ROBERTO CARMONA, KATHLEEN E. CLARK, NIGEL A. CLARK, CARMEN ESPOZ, PATRICIA M. GONZÁLEZ, BRIAN A. HARRINGTON, DANIEL E. HERNÁNDEZ, KEVIN S. KALASZ, RICHARD G. LATHROP, RICARDO N. MATUS, CLIVE D. T. MINTON, R. I. GUY MORRISON, ) Niles et al. Studies in Avian Biology No. 36 MARK K. PECK, WILLIAM PITTS, ROBERT A. ROBINSON, AND INÊS L. SERRANO Studies in Avian Biology No. 36 A Publication of the Cooper Ornithological Society STATUS OF THE RED KNOT (CALIDRIS CANUTUS RUFA) IN THE WESTERN HEMISPHERE Lawrence J. Niles, Humphrey P. Sitters, Amanda D. Dey, Philip W. Atkinson, Allan J. Baker, Karen A. Bennett, Roberto Carmona, Kathleen E. Clark, Nigel A. Clark, Carmen Espoz, Patricia M. González, Brian A. Harrington, Daniel E. Hernández, Kevin S. Kalasz, Richard G. Lathrop, Ricardo N. Matus, Clive D. T. Minton, R. I. Guy Morrison, Mark K. Peck, William Pitts, Robert A. Robinson, and Inês L. Serrano Studies in Avian Biology No. 36 A PUBLICATION OF THE COOPER ORNITHOLOGICAL SOCIETY Front cover photograph of Red Knots by Irene Hernandez Rear cover photograph of Red Knot by Lawrence J. Niles STUDIES IN AVIAN BIOLOGY Edited by Carl D. Marti 1310 East Jefferson Street Boise, ID 83712 Spanish translation by Carmen Espoz Studies in Avian Biology is a series of works too long for The Condor, published at irregular intervals by the Cooper Ornithological Society. -

Red Knot BW Fact Sheet
NT OF E TH TM E R I A N P T E E R D I . O U.S. Fish & Wildlife Service S R . U M A 49 R CH 3, 18 Red knot Calidris canutus rufa Skilled aviator Rear Admiral Richard E. Byrd flew over both the North and South poles. But what this renowned man accomplished with the help of sled dogs, ships and airplanes, a little shorebird weighing less than a cup of coffee completes every year of its life. The red knot is truly a master of long-distance aviation. On wingspans of 20 inches, red knots fly more than 9,300 miles from south to north every spring and repeat the trip in reverse every autumn, making this bird one of the longest-distance migrants in the animal kingdom. About 9 inches long, red knots are among the largest of the small sandpipers. Biologists have identified five races of red knot, three of them living in the Western Hemisphere: C.c. islandica, C.c. rogersi, and C.c. rufa. This last, the red knot known as rufa, winters at the tip Strength in numbers Eating like a bird of South America in Tierra del Fuego and Red knots migrate in larger flocks than In order to endure their long journeys, breeds on the mainland and islands above do most other shorebirds. They break red knots undergo extensive the Arctic Circle. their spring and fall migrations into non- physiological changes. Flight muscle stop segments of 1,500 miles and more, mass increases, while leg muscle mass Surveys of wintering knots along the ending at stopover sites called staging decreases. -

First Results Using Light Level Geolocators to Track Red Knots in the Western Hemisphere Show Rapid and Long Intercontinental F
First results using light level geolocators to track Red Knots in the Western Hemisphere show rapid and long intercontinental flights and new details of migration pathways LAWRENCE J. NILES1, JOANNA BURGER2, RONALD R. PORTER3, AMANDA D. DEY4, CLIVE D.T. MINTON5, PATRICIA M. GONZALEZ6, ALLAN J. BAKER7, JAMES W. FOX8 & CALEB GORDON9 1 Conserve Wildlife Foundation of NJ, 109 Market Lane, Greenwich, NJ 08323, USA. [email protected] 2 Division of Life Sciences, Rutgers University, 604 Allison Road, Piscataway, NJ, USA 3 800 Quinard Court, Ambler, PA, 19002, USA 4 Endangered and Nongame Program, Fish and Wildlife, New Jersey Department of Environmental Protection, 501 E. State Street, PO 420, Trenton, NJ 08625-0420, USA 5 Victorian Wader Study Group, 165 Dalgetty Road, Beaumaris, Melbourne, Victoria 3193, Australia 6 Global Flyway Network, Pedro Morón 385, (8520) San Antonio Oeste, Río Negro, Argentina 7 Royal Ontario Museum, Department of Natural History, 100 Queen’s Park, Toronto, Ontario M5S 2C6, Canada 8 British Antarctic Survey, High Cross, Madingley Road, Cambridge, CB3 0ET, UK 9 Pandion Systems, Inc. 102 NE 10th Ave. Gainesville, FL. 32601, USA Niles, L.J., Burger, J., Porter, R.R., Dey, A.D., Minton, C.D.T., Gonzalez, P.M., Baker, A.J., Fox, J.W. & Gordon, C. 2010. First results using light level geolocators to track Red Knots in the Western Hemisphere show rapid and long intercontinental flights and new details of migration pathways.Wader Study Group Bull. 117(2): 123–130. Keywords: migration, migratory pathways, stopovers, wintering area, breeding area, geolocator, Red Knot, Calidris canutus Geolocators affixed to Darvic leg flags were attached to the tibia of 47 Red KnotsCalidris canutus rufa during the 2009 spring migratory stopover in Delaware Bay, New Jersey, United States. -

Abundance and Distribution of Shorebirds in the San Francisco Bay Area
WESTERN BIRDS Volume 33, Number 2, 2002 ABUNDANCE AND DISTRIBUTION OF SHOREBIRDS IN THE SAN FRANCISCO BAY AREA LYNNE E. STENZEL, CATHERINE M. HICKEY, JANET E. KJELMYR, and GARY W. PAGE, Point ReyesBird Observatory,4990 ShorelineHighway, Stinson Beach, California 94970 ABSTRACT: On 13 comprehensivecensuses of the San Francisco-SanPablo Bay estuaryand associatedwetlands we counted325,000-396,000 shorebirds (Charadrii)from mid-Augustto mid-September(fall) and in November(early winter), 225,000 from late Januaryto February(late winter); and 589,000-932,000 in late April (spring).Twenty-three of the 38 speciesoccurred on all fall, earlywinter, and springcounts. Median counts in one or moreseasons exceeded 10,000 for 10 of the 23 species,were 1,000-10,000 for 4 of the species,and were less than 1,000 for 9 of the species.On risingtides, while tidal fiats were exposed,those fiats held the majorityof individualsof 12 speciesgroups (encompassing 19 species);salt ponds usuallyheld the majorityof 5 speciesgroups (encompassing 7 species); 1 specieswas primarilyon tidal fiatsand in other wetlandtypes. Most speciesgroups tended to concentratein greaterproportion, relative to the extent of tidal fiat, either in the geographiccenter of the estuaryor in the southernregions of the bay. Shorebirds' densitiesvaried among 14 divisionsof the unvegetatedtidal fiats. Most species groups occurredconsistently in higherdensities in someareas than in others;however, most tidalfiats held relativelyhigh densitiesfor at leastone speciesgroup in at leastone season.Areas supportingthe highesttotal shorebirddensities were also the ones supportinghighest total shorebird biomass, another measure of overallshorebird use. Tidalfiats distinguished most frequenfiy by highdensities or biomasswere on the east sideof centralSan FranciscoBay andadjacent to the activesalt ponds on the eastand southshores of southSan FranciscoBay and alongthe Napa River,which flowsinto San Pablo Bay. -

Seven Deadly Stints and Their Friends an Introduction to Calidris Sandpipers – Part 1 Jon L
Seven Deadly Stints and their Friends An Introduction to Calidris Sandpipers – Part 1 Jon L. Dunn Larry Sansone photos 13 October 2020 Los Angeles Birders Genus Calidris – Composed of 23 species the largest genus within the large family (94 species worldwide, 66 in North America) of Scolopacidae (Sandpipers). – All 23 species in the genus Calidris have been found in North America, 19 of which have occurred in California. – Only Great Knot, Broad-billed Sandpiper, Temminck’s Stint, and Spoon-billed Sandpiper have not been recorded in the state, and as for Great Knot, well half of one turned up! Genus Calidris – The genus was described by Marrem in 1804 (type by tautonymy, Red Knot, 1758 Linnaeus). – Until 1934, the genus was composed only of the Red Knot and Great Knot. – This genus is composed of small to moderate sized sandpipers and use a variety of foraging styles from probing in water to picking at the shore’s edge, or even away from water on mud or the vegetated border of the mud. – As within so many families or large genre behavior offers important clues to species identification. Genus Calidris – Most, but not all, species migrate south in their alternate (breeding) or juvenal plumage, molting largely once they reach their more southerly wintering grounds. – Most species nest in the arctic, some farther north than others. Some species breed primarily in Eurasia, some in North America. Some are Holarctic. – The majority of species are monotypic (no additional recognized subspecies). Genus Calidris – In learning these species one -

Migratory Birds Initiative Red Knot, a Priority Knot, Red Species for AMBI 2015-2019
Arctic Migratory Birds Initiative Red knot, a priorityRed species for AMBI 2015-2019. Arendal Prokosch/UNEP-GRID Peter Photo: Arctic Migratory Birds Initiative (AMBI): protecting Arctic lifestyles and peoples through migratory bird conservation is a project designed to improve the status and secure the long-term sustainability of rapidly declining Arctic breeding migratory bird populations. AMBI has developed the Arctic Migratory Birds Initiative Work Plan 2015-2019 to identify priorities and guide actions. Circumpolar Ivory gull • Thick-billed murre • Steller’s eider • Common eider • Long-tailed duck • Snowy owl Americas African-Eurasian Red knot • Semipalmated sandpiper Black-tailed godwit • Bar-tailed godwit • Dunlin • Lesser white-fronted goose • Red knot AMBI works across four major flyways. East Asian-Australasian Each flyway has a series of priority conservation issues and species to Spoon-billed sandpiper • Great knot • Bar-tailed godwit address. • Lesser white-fronted goose • Dunlin • Red knot • The Arctic Council AMBI implementation AMBI responds to the To conserve declining Ministers welcomed will help governments Arctic Biodivesrity bird populations AMBI the AMBI Work Plan meet commitments Assessment welcomes support in the 2015 Arctic under Multilateral recommendation to and collaboration Council Iqaluit Environmental protect migratory with Arctic Council Declaration. Agreements (MEAs) at species range-wide Observers, non- global and hemispheric during the whole year. Arctic States, and levels. international partners. Priority AMBI Conservation Actions and Species Important breeding and staging sites for Spoon-billed Sandpiper, Spoon-billed Sandpiper, a priority species for AMBI 2015-2019. Photo: Jochen Dierschke Bar-tailed Godwit and Dunlin need to be identified and protectedin Arctic Alaska and Russia. -

54971 GPNC Shorebirds
A P ocket Guide to Great Plains Shorebirds Third Edition I I I By Suzanne Fellows & Bob Gress Funded by Westar Energy Green Team, The Nature Conservancy, and the Chickadee Checkoff Published by the Friends of the Great Plains Nature Center Table of Contents • Introduction • 2 • Identification Tips • 4 Plovers I Black-bellied Plover • 6 I American Golden-Plover • 8 I Snowy Plover • 10 I Semipalmated Plover • 12 I Piping Plover • 14 ©Bob Gress I Killdeer • 16 I Mountain Plover • 18 Stilts & Avocets I Black-necked Stilt • 19 I American Avocet • 20 Hudsonian Godwit Sandpipers I Spotted Sandpiper • 22 ©Bob Gress I Solitary Sandpiper • 24 I Greater Yellowlegs • 26 I Willet • 28 I Lesser Yellowlegs • 30 I Upland Sandpiper • 32 Black-necked Stilt I Whimbrel • 33 Cover Photo: I Long-billed Curlew • 34 Wilson‘s Phalarope I Hudsonian Godwit • 36 ©Bob Gress I Marbled Godwit • 38 I Ruddy Turnstone • 40 I Red Knot • 42 I Sanderling • 44 I Semipalmated Sandpiper • 46 I Western Sandpiper • 47 I Least Sandpiper • 48 I White-rumped Sandpiper • 49 I Baird’s Sandpiper • 50 ©Bob Gress I Pectoral Sandpiper • 51 I Dunlin • 52 I Stilt Sandpiper • 54 I Buff-breasted Sandpiper • 56 I Short-billed Dowitcher • 57 Western Sandpiper I Long-billed Dowitcher • 58 I Wilson’s Snipe • 60 I American Woodcock • 61 I Wilson’s Phalarope • 62 I Red-necked Phalarope • 64 I Red Phalarope • 65 • Rare Great Plains Shorebirds • 66 • Acknowledgements • 67 • Pocket Guides • 68 Supercilium Median crown Stripe eye Ring Nape Lores upper Mandible Postocular Stripe ear coverts Hind Neck Lower Mandible ©Dan Kilby 1 Introduction Shorebirds, such as plovers and sandpipers, are a captivating group of birds primarily adapted to live in open areas such as shorelines, wetlands and grasslands. -
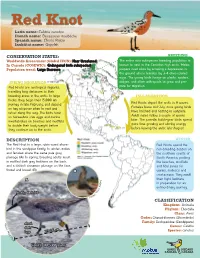
Red Knot Latin Name: Calidris Canutus French Name: Bécasseau Maubèche Spanish Name: Chorlo Rojizo Inuktitut Name: Qajorlak
Red Knot Latin name: Calidris canutus French name: Bécasseau maubèche Spanish name: Chorlo Rojizo Inuktitut name: Qajorlak CONSERVATION STATUS: BREEDING Worldwide Assessment (Global IUCN): Near threatened The entire rufa subspecies breeding population is In Canada (COSEWIC): Endangered (rufa subspecies) known to nest in the Canadian high arctic. Males Population trend: Large Decrease prepare nest sites by scraping a depression in the ground where females lay 3-4 olive-colored eggs. The young birds forage on plants, spiders, SPRING MIGRATION midges, and other arthropods to grow and pre- Red Knots are neotropical migrants, pare for migration. travelling long distances to their breeding areas in the arctic. In large FALL MIGRATION flocks, they begin their 15,000 km journey in late February, and depend Red Knots depart the arctic in 3 waves. on key stopover sites to rest and Females leave mid-July, once young birds refuel along the way. The birds feast have hatched and nesting is complete. on horseshoe crab eggs and marine Adult males follow a couple of weeks invertebrates on beaches and mudflats later. The juvenile, hatch-year birds spend to double their body weight before more time growing and storing energy they continue on to the arctic. before leaving the arctic late-August. DESCRIPTION WINTER The Red Knot is a large, robin-sized shore- Red Knots spend the bird in the sandpiper family. In winter, males non-breeding season on and females share the same pale gray the southern coasts of plumage (A). In spring, breeding adults moult South America, probing in mottled dark gray feathers on the back, the beaches, mudflats and a distinct cinnamon plumage on the face, and tidal zones for throat and breast (B). -

Research on Bar-Tailed Godwits Limosa Lapponica
Texel, November 2017 Research on Bar-tailed Godwits Limosa lapponica In 2001 the Royal Dutch Institute for Sea Research (Royal NIOZ) launched a study on the ecology of the bar-tailed godwit. In addition to the mainly shellfish eating red knot Calidris canutus, which we have been studying since the late eighties, we want to learn more about the ecology of a species whose food consists mainly of bristle worms. In May 2001 a project started with color-rings on bar- tailed godwits in the Dutch Wadden Sea and the Banc d'Arguin in Mauritania, one of the major wintering areas of the species in Western Africa. Every year, birds are caught and ringed by ringers of VRS Castricum (on the Dutch North Sea coast), VRS Calidris on the island Schiermonnikoog and wilsterflappers Joop Jukema, Catharinus Monkel, Jaap Strikwerda and Bram van der Veen. These wilsterflappers are ringing on the islands of Texel, Terschelling and Ameland and catch hundreds of bar-tailed godwits in the meadows each spring. They do this with a traditional catching method that they used in the past to catch golden plovers for their income (wilsterflappen). Waders are also caught by the NIOZ with mist nets in the periods around the new moon in the Wadden Sea and in Mauritania. The color ring combination consists of four color-rings and a flag (a ring with a kind of streamer). The flag colors that have been used since 2001 are shown in Figure 2 and are in chronological order, Yellow (Y), Red (R), Lime (L) and Black (N). -

Red Knot Endangered STATUS Endangered Nova Scotia Calidris Canutus Rufa
9 Red Knot Endangered STATUS Endangered Nova Scotia Calidris canutus rufa Fewer than 15, 000 of the rufa subspecies are left in the wild. Some visit coastal Nova Scotia during migration in the summer and fall. Winters in southern South America. Population Range Habitat Their wintering grounds and habitat during migration consist of coastal areas with large sandflats or mudflats, where they can feed on invertebrates. Peat banks, salt marshes, brackish lagoons and mussel beds are also visited. They breed in the arctic in barren habitats like windswept ridges, slopes and plateaus. Y E L S A L A G D E A R N G A C © S K R A P Species Description , L L L L I I H H R R E E V The Red Knot, rufa subspecies, is a medium-sized (25-28 cm) shorebird V A A C C N N A with a small head and straight, thin bill. In their non-breeding plumage, A N N N N E E R they have a light grey back (with white feather edges), grey-brown breast R B B © © streaks, white underparts and grey legs. Juveniles are similar in appearance but have a black band along the inside of the white feather edge, buffy Red Knots migrate through Nova Scotia along the coast in the summer underparts, and green-yellow legs. In their breeding plumage, they have a and fall. Adults in faded breeding plumage are observed in July and August, brilliant chestnut red breast, neck and face, white underparts, dark legs and a brown back with reddish, tan and black streaks.