Some CSEWG Recollections
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Secrets Jeremy Bernstein
INFERENCE / Vol. 6, No. 1 Secrets Jeremy Bernstein Restricted Data: The History of Nuclear Secrecy in the decided to found a rival weapons laboratory. Even if Teller United States had offered me a job, I doubt that I would have accepted.3 by Alex Wellerstein After obtaining my degree, I was offered a job that University of Chicago Press, 528 pp., $35.00. would keep me in Cambridge for at least another year. One year became two and at the end of my second year I was uclear weapons have been shrouded in secrecy accepted at the Institute for Advanced Study in Princeton. from the very beginning. After plutonium was It was around this time that the chairman of the physics discovered at the University of California in department at Harvard, Kenneth Bainbridge, came to me NDecember 1940, researchers led by Glenn Seaborg submit- with an offer. Bainbridge had been an important figure at ted a pair of letters to the Physical Review. The details of Los Alamos during the war. Robert Oppenheimer had put their discovery were withheld from publication until after him in charge of the site in New Mexico where the Trinity the war.1 Once the project to make a nuclear weapon got test had taken place.4 Bainbridge told me that the labora- underway, secrecy became a very serious matter indeed. tory was offering summer jobs to young PhDs and asked The story of these efforts and how they evolved after the if I was interested. I was very interested. Los Alamos had war is the subject of Alex Wellerstein’s Restricted Data: an almost mystical significance for me due to its history The History of Nuclear Secrecy in the United States. -

Development of an On-Line Fuel Failure Monitoring System For
DEVELOPMENT OF AN ON-LINE FUEL FAILURE MONITORING SYSTEM FOR CANDU REACTORS DEVELOPPEMENT D'UN SYSTEME DE SURVEILLANCE EN LIGNE POUR DES RUPTURES DE GAINES DES REACTEURS CANDU A Thesis Submitted to the Division of Graduate Studies of the Royal Military College of Canada by Stephen Jason Livingstone, BSc, MSc Sub-Lieutenant In Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy March 2012 ©This thesis may be used within the Department of National Defence but copyright for open publication remains the property of the author. Library and Archives Bibliotheque et Canada Archives Canada Published Heritage Direction du Branch Patrimoine de I'edition 395 Wellington Street 395, rue Wellington Ottawa ON K1A0N4 Ottawa ON K1A 0N4 Canada Canada Your file Votre reference ISBN: 978-0-494-83407-7 Our file Notre reference ISBN: 978-0-494-83407-7 NOTICE: AVIS: The author has granted a non L'auteur a accorde une licence non exclusive exclusive license allowing Library and permettant a la Bibliotheque et Archives Archives Canada to reproduce, Canada de reproduire, publier, archiver, publish, archive, preserve, conserve, sauvegarder, conserver, transmettre au public communicate to the public by par telecommunication ou par Plnternet, preter, telecommunication or on the Internet, distribuer et vendre des theses partout dans le loan, distrbute and sell theses monde, a des fins commerciales ou autres, sur worldwide, for commercial or non support microforme, papier, electronique et/ou commercial purposes, in microform, autres formats. paper, electronic and/or any other formats. The author retains copyright L'auteur conserve la propriete du droit d'auteur ownership and moral rights in this et des droits moraux qui protege cette these. -
![小型飛翔体/海外 [Format 2] Technical Catalog Category](https://docslib.b-cdn.net/cover/2534/format-2-technical-catalog-category-112534.webp)
小型飛翔体/海外 [Format 2] Technical Catalog Category
小型飛翔体/海外 [Format 2] Technical Catalog Category Airborne contamination sensor Title Depth Evaluation of Entrained Products (DEEP) Proposed by Create Technologies Ltd & Costain Group PLC 1.DEEP is a sensor analysis software for analysing contamination. DEEP can distinguish between surface contamination and internal / absorbed contamination. The software measures contamination depth by analysing distortions in the gamma spectrum. The method can be applied to data gathered using any spectrometer. Because DEEP provides a means of discriminating surface contamination from other radiation sources, DEEP can be used to provide an estimate of surface contamination without physical sampling. DEEP is a real-time method which enables the user to generate a large number of rapid contamination assessments- this data is complementary to physical samples, providing a sound basis for extrapolation from point samples. It also helps identify anomalies enabling targeted sampling startegies. DEEP is compatible with small airborne spectrometer/ processor combinations, such as that proposed by the ARM-U project – please refer to the ARM-U proposal for more details of the air vehicle. Figure 1: DEEP system core components are small, light, low power and can be integrated via USB, serial or Ethernet interfaces. 小型飛翔体/海外 Figure 2: DEEP prototype software 2.Past experience (plants in Japan, overseas plant, applications in other industries, etc) Create technologies is a specialist R&D firm with a focus on imaging and sensing in the nuclear industry. Createc has developed and delivered several novel nuclear technologies, including the N-Visage gamma camera system. Costainis a leading UK construction and civil engineering firm with almost 150 years of history. -

Cold War Requisitions, Scientific Manpower, and the Production of American Physicists After World War II
DAVID KAISER* Cold War requisitions, scientific manpower, and the production of American physicists after World War II 1. RAYMOND BIRGE’S “MAIN OBJECTIVE” “THE MAIN OBJECTIVE of this department of physics,” Raymond Birge wrote in late May 1955, “is to train Ph.D.’s in physics.” Birge— iconic, somber, a displaced Yankee who traced his New England ancestry nine generations back—had been chair of Berkeley’s physics department for twenty-two years; by the mid-1950s, it was the nation’s largest. At the time he explained his department’s “main objec- tive,” Birge was the retiring president of the American Physical Society (APS). Birge and his colleagues in Berkeley’s physics department had emphasized the importance of its graduate program many times before in annual budget requests to the university administration and in funding reports to private industries; it would be easy to read such remarks as thinly-veiled requests for more funding, since training physics Ph.D.s became expensive after World War II. This time, however, Birge articulated his department’s mission in a letter to a local citizen, far outside of the university bureaucracy, who had no funds to offer and who had requested no such pronouncement. 1 *Program in Science, Technology, and Society, and Department of Physics, Building E51- 185, MIT, Cambridge, MA 02139; [email protected]. My thanks to Shane Hamilton for his research assistance, and to Alexis De Greiff, Kenji Ito, John Krige, Elizabeth Paris, and John Rudolph for their helpful comments on an earlier draft. The following abbreviations are used: AIP-EMD, American Institute of Physics, Edu- cation and Manpower Division Records, Niels Bohr Library, American Institute of Physics, College Park, MD; BAS, Bulletin of the atomic scientists; BDP , University of California, Berkeley, Department of Physics Records, Bancroft Library, Berkeley, CA; HDP, Harvard University Department of Physics Records, Pusey Library, Cambridge, MA; PDP, Princeton University Department of Physics Records, Seeley G. -

Study of Macromolecule-Mineral Interactions on Nuclear Related Materials
Study of macromolecule-mineral interactions on nuclear related materials by Lygia Eleftheriou Submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy Supervised by: Prof John Harding Dr Maria Romero-González The University of Sheffield Faculty of Engineering Department of Materials Science and Engineering September 2016 Declaration The work described within this thesis has been completed under the supervision of Prof J. Harding and Dr M. Romero-González at the University of Sheffield between September 2012 and September 2016. This thesis along with the work described here has been completed by the author with some exceptions indicated clearly at the relevant chapters. These include: (1) the construction of ceria models for the computational work that was completed by Dr Colin Freeman and Dr Shaun Hall (described in chapter 5), (2) the purification of peptidoglycan completed by Dr Stephane Mesnage (described in chapter 4) and (3) the electron microscopy analysis completed by Dr Mohamed Merroun (described in chapter 2). Lygia Eleftheriou September 2016 Acknowledgements I would like to express my sincere gratitude to my supervisors Dr Maria Romero González and Prof John Harding for all their support during the past four years. This work would not have been possible without their endless encouragement, guidance and advice. I would also like to thank Dr Colin Freeman, Dr Shaun Hall and Riccardo Innocenti Malini for all the hours they spent trying to make things work and all their help with the computational part of this project. In addition, I would like to thank Dr Simon Thorpe, Dr Stephane Mesnage and Mr Robert Hanson for their help with the analytical methods of this project. -

THE MEETING Meridel Rubenstein 1995
THE MEETING Meridel Rubenstein 1995 Palladium prints, steel, single-channel video Video assistance by Steina Video run time 4:00 minutes Tia Collection The Meeting consists of twenty portraits of people from San Ildefonso Pueblo and Manhattan Project physicists—who met at the home of Edith Warner during the making of the first atomic bomb—and twenty photographs of carefully selected objects of significance to each group. In this grouping are people from San Ildefonso Pueblo and the objects they selected from the collections of the Museum of Indian Arts and Culture to represent their culture. 1A ROSE HUGHES 2A TALL-NECKED JAR 3A BLUE CORN 4A SLEIGH BELLS 5A FLORENCE NARANJO Rose Hughes holding a photograph of WITH AVANYU One of the most accomplished and (Museum of Indian Arts and Culture) Married to Louis Naranjo; her father, Tony Peña, who organized (plumed serpent) made by Julian and recognized of the San Ildefonso Sleigh bells are commonly used in granddaughter of Ignacio and Susana the building of Edith Warner’s second Maria Martinez, ca. 1930 (Museum of potters. Like many women from the ceremonial dances to attract rain. Aguilar; daughter of Joe Aguilar, who house. Hughes worked at Edith Indian Arts and Culture) Edith Warner pueblos, she worked as a maid for the Tilano Montoya returned with bells like helped Edith Warner remodel the Warner’s with Florence Naranjo one was shown a pot like this one in 1922 Oppenheimers. these from Europe, where he went on tearoom. Edith called her Florencita. summer. She recalls that Edith once on her first visit to San Ildefonso, in the tour with a group of Pueblo dancers. -

Physics 111 Fall 2007 Radioactive Decay Problems Solutions
Physics 111 Fall 2007 Radioactive Decay Problems Solutions 3 1. The 1 H isotope of hydrogen, which is called tritium (because it contains three nucleons), has a half-life of 12.33 yr. It can be used to measure the age of objects up to about 100 yr. It is produced in the upper atmosphere by cosmic rays and brought to Earth by rain. As an application, determine approximately the age of a bottle of wine 3 1 whose 1 H radiation is about 10 that present in new wine. Because the tritium in water is being replenished, we assume that the amount is constant until the wine is made, and then it decays. We find the number of half-lives from N n = 1 ; N ()2 0 n 0.10 = 1 , or n log 2 = log 10 , which gives n = 3.32. ()2 () Thus the time is t = nT = 3.32 12.33 yr = 41 yr. 1 ()() 2 2. Strontium-90 is produced as a nuclear fission product of uranium in both reactors and atomic bombs. Look at its location in the periodic table to see what other elements it might be similar to chemically, and tell why you think it might be dangerous to ingest. It has too many neutrons, and it decays with a half-life of about 29 yr. How 90 long will we have to wait for the amount of 38 Sr on the Earth’s surface to reach 1% of its current level, assuming no new material is scattered about? Write down the decay reaction, including the daughter nucleus. -

Trinity Site July 16, 1945
Trinity Site July 16, 1945 "The effects could well be called unprecedented, magnificent, beauti ful, stupendous, and terrifying. No man-made phenomenon of such tremendous power had ever occurred before. The lighting effects beggared description. The whole country was lighted by a searing light with the intensity many times that of the midday sun." Brig. Gen. Thomas Farrell A national historic landmark on White Sands Missile Range -- www.wsmr.army.mil Radiation Basics Radiation comes from the nucJeus of the gamma ray. This is a type of electromag individual atoms. Simple atoms like oxygen netic radiation like visible light, radio waves are very stable. Its nucleus has eight protons and X-rays. They travel at the speed of light. and eight neutrons and holds together well. It takes at least an inch of lead or eight The nucJeus of a complex atom like inches of concrete to stop them. uranium is not as stable. Uranium has 92 Finally, neutrons are also emitted by protons and 146 neutrons in its core. These some radioactive substances. Neutrons are unstable atoms tend to break down into very penetrating but are not as common in more stable, simpler forms. When this nature. Neutrons have the capability of happens the atom emits subatomic particles striking the nucleus of another atom and and gamma rays. This is where the word changing a stable atom into an unstable, and "radiation" comes from -- the atom radiates therefore, radioactive one. Neutrons emitted particles and rays. in nuc!ear reactors are contained in the Health physicists are concerned with reactor vessel or shielding and cause the four emissions from the nucleus of these vessel walls to become radioactive. -

Perspective the Radioactivity of Atmospheric Krypton in 1949–1950
Proc. Natl. Acad. Sci. USA Vol. 94, pp. 7807–7810, July 1997 Perspective The radioactivity of atmospheric krypton in 1949–1950 Anthony Turkevich*, Lester Winsberg†, Howard Flotow‡, and Richard M. Adams§ Argonne National Laboratory, 9700 S. Cass Avenue, Argonne, IL 60439 Contributed by Anthony Turkevich, April 10, 1997 ABSTRACT The chemical element krypton, whose prin- Libby (in 1948; unpublished work) gave indications that these cipal source is the atmosphere, had a long-lived radioactive predictions were correct. The present report describes the content, in the mid-1940s, of less than 5 dpm per liter of technique that was used soon afterward to establish more krypton. In the late 1940s, this content had risen to values in precisely that atmospheric krypton in the late 1940s was much the range of 100 dpm per liter. It is now some hundred times more radioactive than it had been, and gives the results of some higher than the late 1940 values. This radioactivity is the early measurements of krypton samples isolated from the result of the dissolving of nuclear fuel for military and civilian atmosphere at that time.** purposes, and the release thereby of the fission product krypton-85 (half-life 5 10.71 years, fission yield 5 0.2%). The Measurement Technique present largest emitter of krypton-85 is the French reprocess- ing plant at Cap-de-la-Hague. The measurement technique that was used was influenced by those simultaneously being developed by Libby and Anderson It is generally known that the chemical element krypton, (9) to measure the radiocarbon content of samples of arche- isolated from the atmosphere in 1996, is radioactive. -
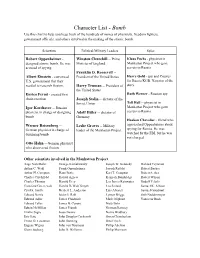
Character List
Character List - Bomb Use this chart to help you keep track of the hundreds of names of physicists, freedom fighters, government officials, and others involved in the making of the atomic bomb. Scientists Political/Military Leaders Spies Robert Oppenheimer - Winston Churchill -- Prime Klaus Fuchs - physicist in designed atomic bomb. He was Minister of England Manhattan Project who gave accused of spying. secrets to Russia Franklin D. Roosevelt -- Albert Einstein - convinced President of the United States Harry Gold - spy and Courier U.S. government that they for Russia KGB. Narrator of the needed to research fission. Harry Truman -- President of story the United States Enrico Fermi - created first Ruth Werner - Russian spy chain reaction Joseph Stalin -- dictator of the Tell Hall -- physicist in Soviet Union Igor Korchatov -- Russian Manhattan Project who gave physicist in charge of designing Adolf Hitler -- dictator of secrets to Russia bomb Germany Haakon Chevalier - friend who Werner Reisenberg -- Leslie Groves -- Military approached Oppenheimer about German physicist in charge of leader of the Manhattan Project spying for Russia. He was designing bomb watched by the FBI, but he was not charged. Otto Hahn -- German physicist who discovered fission Other scientists involved in the Manhattan Project: Aage Niels Bohr George Kistiakowsky Joseph W. Kennedy Richard Feynman Arthur C. Wahl Frank Oppenheimer Joseph Rotblat Robert Bacher Arthur H. Compton Hans Bethe Karl T. Compton Robert Serber Charles Critchfield Harold Agnew Kenneth Bainbridge Robert Wilson Charles Thomas Harold Urey Leo James Rainwater Rudolf Pelerls Crawford Greenewalt Harold DeWolf Smyth Leo Szilard Samuel K. Allison Cyril S. Smith Herbert L. Anderson Luis Alvarez Samuel Goudsmit Edward Norris Isidor I. -

LRB · Steven Shapin: Don't Let That Crybaby in Here Again
LRB · Steven Shapin: Don’t let that crybaby in here again http://www.lrb.co.uk/v22/n17/print/shap01_.html HOME SUBSCRIBE LOG OUT CONTACTS SEARCH LRB 7 September 2000 Steven Shapin screen layout tell a friend Don’t let that crybaby in here again Steven Shapin In the Shadow of the Bomb: Oppenheimer, Bethe and the Moral Responsibility of the Scientist by S.S. Schweber Atomic Fragments: A Daughter’s Questions by Mary Palevsky The rhetorical yield from the first atomic explosion was low – only one entry for the Oxford Dictionary of Quotations. When the plutonium bomb exploded on the Jornada del Muerto near Alamogordo, New Mexico, on 16 July 1945, Robert Oppenheimer, the Scientific Director of Los Alamos, remembered the line from the Bhagavad Gita where Vishnu says: ‘Now I am become Death, the destroyer of worlds.’ One other remark deserves to be immortalised, which Oppenheimer himself later judged the best thing said at the time. When the blast subsided, the physicist Kenneth Bainbridge, in charge of the test, turned to Oppenheimer and declared: ‘Now we’re all sons of bitches.’ In general, however, the test was a rhetorical dud. After the physicist Samuel Allison had counted off ‘two, one, zero, NOW,’ a general standing by commented: ‘What a wonderful thing that you could count backwards at a time like this!’ Allison recalled saying to himself: ‘Still alive, no atmospheric ignition.’ The chemist George Kistiakowsky rushed up to Oppenheimer to remind him of a bet they’d struck on the outcome: ‘Oppie, you owe me ten dollars.’ General Leslie Groves, the overall Director of the Manhattan Project, immediately appreciated the military significance of what he’d just seen: ‘The bang must certainly have been a pretty big one . -

Physics, Physicists and the Bomb
editorial Physics, physicists and the bomb Scientists involved in nuclear research before and after the end of the Second World War continue to be the subjects of historical and cultural fascination. Almost 70 years since Hiroshima and Nagasaki, the military, historical and moral implications of the nuclear bomb remain firmly lodged in the public’s consciousness. Images of mushroom clouds serve as powerful reminders of the destructive capability that countries armed with nuclear weapons have access to — a capability that continues to play a primary role in shaping the present geopolitical landscape of the world. For physicists, the development of the nuclear bomb generally brings up conflicting feelings. On the one hand, physicists played a central role in helping to create it; on the SCIENCE SOURCE/SCIENCE PHOTO LIBRARY PHOTO SOURCE/SCIENCE SCIENCE other, they were also among the first to realize © its terrifying power. This contradiction is most Manhattan Project physicists at Los Alamos. From left to right: Kenneth Bainbridge, Joseph Hoffman, famously epitomized by Robert Oppenheimer, Robert Oppenheimer, Louis Hempelmann, Robert Bacher, Victor Weisskopf and Richard Dodson. the scientific director of the Manhattan Project, who, on witnessing the first test of the atomic bomb, the Trinity test, in July 1945, in this context that the public can truly come race following the Second World War, there was reminded of a quote from the Hindu to feel the growing sense of disillusionment is no question that Churchill was an early scripture Bhagavad Gita: “Now, I am become of those scientists as they realized their goal; and influential champion for government- Death, the destroyer of worlds.” a sense of lost innocence, that knowledge that sponsored science and technology in Britain.