Clouds and Turbulent Moist Convection
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Soaring Weather
Chapter 16 SOARING WEATHER While horse racing may be the "Sport of Kings," of the craft depends on the weather and the skill soaring may be considered the "King of Sports." of the pilot. Forward thrust comes from gliding Soaring bears the relationship to flying that sailing downward relative to the air the same as thrust bears to power boating. Soaring has made notable is developed in a power-off glide by a conven contributions to meteorology. For example, soar tional aircraft. Therefore, to gain or maintain ing pilots have probed thunderstorms and moun altitude, the soaring pilot must rely on upward tain waves with findings that have made flying motion of the air. safer for all pilots. However, soaring is primarily To a sailplane pilot, "lift" means the rate of recreational. climb he can achieve in an up-current, while "sink" A sailplane must have auxiliary power to be denotes his rate of descent in a downdraft or in come airborne such as a winch, a ground tow, or neutral air. "Zero sink" means that upward cur a tow by a powered aircraft. Once the sailcraft is rents are just strong enough to enable him to hold airborne and the tow cable released, performance altitude but not to climb. Sailplanes are highly 171 r efficient machines; a sink rate of a mere 2 feet per second. There is no point in trying to soar until second provides an airspeed of about 40 knots, and weather conditions favor vertical speeds greater a sink rate of 6 feet per second gives an airspeed than the minimum sink rate of the aircraft. -

Weather Charts Natural History Museum of Utah – Nature Unleashed Stefan Brems
Weather Charts Natural History Museum of Utah – Nature Unleashed Stefan Brems Across the world, many different charts of different formats are used by different governments. These charts can be anything from a simple prognostic chart, used to convey weather forecasts in a simple to read visual manner to the much more complex Wind and Temperature charts used by meteorologists and pilots to determine current and forecast weather conditions at high altitudes. When used properly these charts can be the key to accurately determining the weather conditions in the near future. This Write-Up will provide a brief introduction to several common types of charts. Prognostic Charts To the untrained eye, this chart looks like a strange piece of modern art that an angry mathematician scribbled numbers on. However, this chart is an extremely important resource when evaluating the movement of weather fronts and pressure areas. Fronts Depicted on the chart are weather front combined into four categories; Warm Fronts, Cold Fronts, Stationary Fronts and Occluded Fronts. Warm fronts are depicted by red line with red semi-circles covering one edge. The front movement is indicated by the direction the semi- circles are pointing. The front follows the Semi-Circles. Since the example above has the semi-circles on the top, the front would be indicated as moving up. Cold fronts are depicted as a blue line with blue triangles along one side. Like warm fronts, the direction in which the blue triangles are pointing dictates the direction of the cold front. Stationary fronts are frontal systems which have stalled and are no longer moving. -

Effect of Wind on Near-Shore Breaking Waves
EFFECT OF WIND ON NEAR-SHORE BREAKING WAVES by Faydra Schaffer A Thesis Submitted to the Faculty of The College of Engineering and Computer Science in Partial Fulfillment of the Requirements for the Degree of Master of Science Florida Atlantic University Boca Raton, Florida December 2010 Copyright by Faydra Schaffer 2010 ii ACKNOWLEDGEMENTS The author wishes to thank her mother and family for their love and encouragement to go to college and be able to have the opportunity to work on this project. The author is grateful to her advisor for sponsoring her work on this project and helping her to earn a master’s degree. iv ABSTRACT Author: Faydra Schaffer Title: Effect of wind on near-shore breaking waves Institution: Florida Atlantic University Thesis Advisor: Dr. Manhar Dhanak Degree: Master of Science Year: 2010 The aim of this project is to identify the effect of wind on near-shore breaking waves. A breaking wave was created using a simulated beach slope configuration. Testing was done on two different beach slope configurations. The effect of offshore winds of varying speeds was considered. Waves of various frequencies and heights were considered. A parametric study was carried out. The experiments took place in the Hydrodynamics lab at FAU Boca Raton campus. The experimental data validates the knowledge we currently know about breaking waves. Offshore winds effect is known to increase the breaking height of a plunging wave, while also decreasing the breaking water depth, causing the wave to break further inland. Offshore winds cause spilling waves to react more like plunging waves, therefore increasing the height of the spilling wave while consequently decreasing the breaking water depth. -

Impacts of Anthropogenic Aerosols on Fog in North China Plain
BNL-209645-2018-JAAM Journal of Geophysical Research: Atmospheres RESEARCH ARTICLE Impacts of Anthropogenic Aerosols on Fog in North China Plain 10.1029/2018JD029437 Xingcan Jia1,2,3 , Jiannong Quan1 , Ziyan Zheng4, Xiange Liu5,6, Quan Liu5,6, Hui He5,6, 2 Key Points: and Yangang Liu • Aerosols strengthen and prolong fog 1 2 events in polluted environment Institute of Urban Meteorology, Chinese Meteorological Administration, Beijing, China, Brookhaven National Laboratory, • Aerosol effects are stronger on Upton, NY, USA, 3Key Laboratory of Aerosol-Cloud-Precipitation of China Meteorological Administration, Nanjing University microphysical properties than of Information Science and Technology, Nanjing, China, 4Institute of Atmospheric Physics, Chinese Academy of Sciences, macrophysical properties Beijing, China, 5Beijing Weather Modification Office, Beijing, China, 6Beijing Key Laboratory of Cloud, Precipitation and • Turbulence is enhanced by aerosols during fog formation and growth but Atmospheric Water Resources, Beijing, China suppressed during fog dissipation Abstract Fog poses a severe environmental problem in the North China Plain, China, which has been Supporting Information: witnessing increases in anthropogenic emission since the early 1980s. This work first uses the WRF/Chem • Supporting Information S1 model coupled with the local anthropogenic emissions to simulate and evaluate a severe fog event occurring Correspondence to: in North China Plain. Comparison of the simulations against observations shows that WRF/Chem well X. Jia and Y. Liu, reproduces the general features of temporal evolution of PM2.5 mass concentration, fog spatial distribution, [email protected]; visibility, and vertical profiles of temperature, water vapor content, and relative humidity in the planetary [email protected] boundary layer throughout the whole period of the fog event. -

NWS Unified Surface Analysis Manual
Unified Surface Analysis Manual Weather Prediction Center Ocean Prediction Center National Hurricane Center Honolulu Forecast Office November 21, 2013 Table of Contents Chapter 1: Surface Analysis – Its History at the Analysis Centers…………….3 Chapter 2: Datasets available for creation of the Unified Analysis………...…..5 Chapter 3: The Unified Surface Analysis and related features.……….……….19 Chapter 4: Creation/Merging of the Unified Surface Analysis………….……..24 Chapter 5: Bibliography………………………………………………….…….30 Appendix A: Unified Graphics Legend showing Ocean Center symbols.….…33 2 Chapter 1: Surface Analysis – Its History at the Analysis Centers 1. INTRODUCTION Since 1942, surface analyses produced by several different offices within the U.S. Weather Bureau (USWB) and the National Oceanic and Atmospheric Administration’s (NOAA’s) National Weather Service (NWS) were generally based on the Norwegian Cyclone Model (Bjerknes 1919) over land, and in recent decades, the Shapiro-Keyser Model over the mid-latitudes of the ocean. The graphic below shows a typical evolution according to both models of cyclone development. Conceptual models of cyclone evolution showing lower-tropospheric (e.g., 850-hPa) geopotential height and fronts (top), and lower-tropospheric potential temperature (bottom). (a) Norwegian cyclone model: (I) incipient frontal cyclone, (II) and (III) narrowing warm sector, (IV) occlusion; (b) Shapiro–Keyser cyclone model: (I) incipient frontal cyclone, (II) frontal fracture, (III) frontal T-bone and bent-back front, (IV) frontal T-bone and warm seclusion. Panel (b) is adapted from Shapiro and Keyser (1990) , their FIG. 10.27 ) to enhance the zonal elongation of the cyclone and fronts and to reflect the continued existence of the frontal T-bone in stage IV. -

GEOGRAPHY OPTIONAL by SHAMIM ANWER
GEOGRAPHY OPTIONAL by SHAMIM ANWER LEARNING GEOGRAPHY - A NEVER BEFORE EXPERIENCE PREP SUPPLEMENT CLIMA T OLOGY NOT FOR SALE | Off : 57/17 1st Floor, Old Rajender Nagar 8026506054, 8826506099 | Above Dr, Batra’s Delhi - 110060 Also visit us on www.keynoteias.com | www.facebook.com/keynoteias.in KEYNOTE IAS PHYSICAL GEOGRAPHY CLIMATOLOGY INDEX 1. CIRCULATION OF THE ATMOSPHERE ................................................................1-13 Local Winds; Observed Distribution of Pressure and Winds and Idealized Zonal Pressure Belts; Monsoons, Westerlies and Jet Streams; EL Nino and La Nina. 2. MOISTURE AND ATMOSPHERIC STABILITY ..................................................14-24 Atmospheric Stability and Instability 3. FORMS OF CONDENSATION AND PRECIPITATION ........................................25-38 Types and Global Distribution of Precipitation 4. AIR MASSES...........................................................................................................39-53 Polar-Front Theory; Fronts; Cyclone Formation; Cyclonic and Anticyclonic Circulation PREP-SUPPLEMENT: CLIMATOLOGY 1 1. CIRCULATION OF THE ATMOSPHERE Atmospheric circulation and wind Macroscale Winds: The largest wind patterns, called macroscale winds, are exemplified by the Winds are generated by pressure differences that westerlies and trade winds. These planetary-scale arise because of unequal heating of Earth's surface. flow patterns extend around the entire globe and Global winds are generated because the tropics can remain essentially unchanged for weeks at -

Evidence of Fire in the Pliocene Arctic in Response to Elevated CO2 and Temperature
1 Evidence of fire in the Pliocene Arctic in response to elevated CO2 and temperature 2 Tamara Fletcher1*, Lisa Warden2*, Jaap S. Sinninghe Damsté2,3, Kendrick J. Brown4,5, Natalia 3 Rybczynski6,7, John Gosse8, and Ashley P Ballantyne1 4 1 College of Forestry and Conservation, University of Montana, Missoula, 59812, USA 5 2 Department of Marine Microbiology and Biogeochemistry, NIOZ Royal Netherlands Institute for Sea Research, Den 6 Berg, 1790, Netherlands 7 3 Department of Earth Sciences, University of Utrecht, Utrecht, 3508, Netherlands 8 4 Natural Resources Canada, Canadian Forest Service, Victoria, V8Z 1M, Canada 9 5 Department of Earth, Environmental and Geographic Science, University of British Columbia Okanagan, Kelowna, 10 V1V 1V7, Canada 11 6 Department of Palaeobiology, Canadian Museum of Nature, Ottawa, K1P 6P4, Canada 12 7 Department of Biology & Department of Earth Sciences, Carleton University, Ottawa, K1S 5B6, Canada 13 8 Department of Earth Sciences, Dalhousie University, Halifax, B3H 4R2, Canada 14 *Authors contributed equally to this work 15 Correspondence to: Tamara Fletcher ([email protected]) 16 Abstract. The mid-Pliocene is a valuable time interval for understanding the mechanisms that determine equilibrium 17 climate at current atmospheric CO2 concentrations. One intriguing, but not fully understood, feature of the early to 18 mid-Pliocene climate is the amplified arctic temperature response. Current models underestimate the degree of 19 warming in the Pliocene Arctic and validation of proposed feedbacks is limited by scarce terrestrial records of climate 20 and environment, as well as discrepancies in current CO2 proxy reconstructions. Here we reconstruct the CO2, summer 21 temperature and fire regime from a sub-fossil fen-peat deposit on west-central Ellesmere Island, Canada, that has been 22 chronologically constrained using radionuclide dating to 3.9 +1.5/-0.5 Ma. -
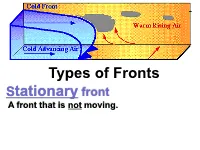
Types of Fronts Stationary Front a Front That Is Not Moving
Types of Fronts Stationary front A front that is not moving. Types of Fronts Cold front is a leading edge of colder air that is replacing warmer air. Types of Fronts Warm front is a leading edge of warmer air that is replacing cooler air. Types of Fronts Occluded front: When a cold front catches up to a warm front. Types of Fronts Dry Line Separates a moist air mass from a dry air mass. A.Cold Front is a transition zone from warm air to cold air. A cold front is defined as the transition zone where a cold air mass is replacing a warmer air mass. Cold fronts generally move from northwest to southeast. The air behind a cold front is noticeably colder and drier than the air ahead of it. When a cold front passes through, temperatures can drop more than 15 degrees within the first hour. The station east of the front reported a temperature of 55 degrees Fahrenheit while a short distance behind the front, the temperature decreased to 38 degrees. An abrupt temperature change over a short distance is a good indicator that a front is located somewhere in between. B. Warm Front. • A transition zone from cold air to warm air. • A warm front is defined as the transition zone where a warm air mass is replacing a cold air mass. Warm fronts generally move from southwest to northeast . The air behind a warm front is warmer and more moist than the air ahead of it. When a warm front passes through, the air becomes noticeably warmer and more humid than it was before. -

Atmospheric Stability Atmospheric Lapse Rate
ATMOSPHERIC STABILITY ATMOSPHERIC LAPSE RATE The atmospheric lapse rate ( ) refers to the change of an atmospheric variable with a change of altitude, the variable being temperature unless specified otherwise (such as pressure, density or humidity). While usually applied to Earth's atmosphere, the concept of lapse rate can be extended to atmospheres (if any) that exist on other planets. Lapse rates are usually expressed as the amount of temperature change associated with a specified amount of altitude change, such as 9.8 °Kelvin (K) per kilometer, 0.0098 °K per meter or the equivalent 5.4 °F per 1000 feet. If the atmospheric air cools with increasing altitude, the lapse rate may be expressed as a negative number. If the air heats with increasing altitude, the lapse rate may be expressed as a positive number. Understanding of lapse rates is important in micro-scale air pollution dispersion analysis, as well as urban noise pollution modeling, forest fire-fighting and certain aviation applications. The lapse rate is most often denoted by the Greek capital letter Gamma ( or Γ ) but not always. For example, the U.S. Standard Atmosphere uses L to denote lapse rates. A few others use the Greek lower case letter gamma ( ). Types of lapse rates There are three types of lapse rates that are used to express the rate of temperature change with a change in altitude, namely the dry adiabatic lapse rate, the wet adiabatic lapse rate and the environmental lapse rate. Dry adiabatic lapse rate Since the atmospheric pressure decreases with altitude, the volume of an air parcel expands as it rises. -

Atmospheric Thermal and Dynamic Vertical Structures of Summer Hourly Precipitation in Jiulong of the Tibetan Plateau
atmosphere Article Atmospheric Thermal and Dynamic Vertical Structures of Summer Hourly Precipitation in Jiulong of the Tibetan Plateau Yonglan Tang 1, Guirong Xu 1,*, Rong Wan 1, Xiaofang Wang 1, Junchao Wang 1 and Ping Li 2 1 Hubei Key Laboratory for Heavy Rain Monitoring and Warning Research, Institute of Heavy Rain, China Meteorological Administration, Wuhan 430205, China; [email protected] (Y.T.); [email protected] (R.W.); [email protected] (X.W.); [email protected] (J.W.) 2 Heavy Rain and Drought-Flood Disasters in Plateau and Basin Key Laboratory of Sichuan Province, Institute of Plateau Meteorology, China Meteorological Administration, Chengdu 610072, China; [email protected] * Correspondence: [email protected]; Tel.: +86-27-8180-4913 Abstract: It is an important to study atmospheric thermal and dynamic vertical structures over the Tibetan Plateau (TP) and their impact on precipitation by using long-term observation at represen- tative stations. This study exhibits the observational facts of summer precipitation variation on subdiurnal scale and its atmospheric thermal and dynamic vertical structures over the TP with hourly precipitation and intensive soundings in Jiulong during 2013–2020. It is found that precipitation amount and frequency are low in the daytime and high in the nighttime, and hourly precipitation greater than 1 mm mostly occurs at nighttime. Weak precipitation during the daytime may be caused by air advection, and strong precipitation at nighttime may be closely related with air convection. Both humidity and wind speed profiles show obvious fluctuation when precipitation occurs, and the greater the precipitation intensity, the larger the fluctuation. Moreover, the fluctuation of wind speed Citation: Tang, Y.; Xu, G.; Wan, R.; Wang, X.; Wang, J.; Li, P. -
QUICK REFERENCE GUIDE XT and XE Ovens
QUICK REFERENCE GUIDE XT AND XE OVENS SYMBOL OVEN FUNCTION FUNCTION SELECTION SETTING BEST FOR Select on the desired Activates the upper and the temperature through the This cooking mode is suitable for any Bake either oven control knob lower heating elements or the touch control smart kind of dishes and it is great for baking botton. 100°F - 500°F and roasting. Food probe allowed. Select on the desired Reduced cooking time (up to 10%). Activates the upper and the temperature through the Ideal for multi-level baking and roasting Convection bake either oven control knob lower heating elements or the touch control smart of meat and poultry. botton. 100°F - 500°F Food probe allowed. Select on the desired Reduced cooking time (up to 10%). Activates the circular heating temperature through the Ideal for multi-level baking and roasting, Convection elements and the convection either oven control knob especially for cakes and pastry. Food fan or the touch control smart probe allowed. botton. 100°F - 500°F Activates the upper heating Select on the desired Reduced cooking time (up to 10%). temperature through the Best for multi-level baking and roasting, Turbo element, the circular heating either oven control knob element and the convection or the touch control smart especially for pizza, focaccia and fan botton. 100°F - 500°F bread. Food probe allowed. Ideal for searing and roasting small cuts of beef, pork, poultry, and for 4 power settings – LOW Broil Activates the broil element (1) to HIGH (4) grilling vegetables. Food probe allowed. Activates the broil element, Ideal for browning fish and other items 4 power settings – LOW too delicate to turn and thicker cuts of Convection broil the upper heating element (1) to HIGH (4) and the convection fan steaks. -

(Highresmip V1.0) for CMIP6
High Resolution Model Intercomparison Project (HighResMIP v1.0) for CMIP6 1 2 3 5 Reindert J. Haarsma , Malcolm Roberts , Pier Luigi Vidale , Catherine A. Senior2, Alessio Bellucci4, Qing Bao5, Ping Chang6, Susanna Corti7, Neven S. Fučkar8, Virginie Guemas8, Jost von Hardenberg7, Wilco Hazeleger1,9,10, Chihiro Kodama11, Torben Koenigk12, Lai-Yung Ruby Leung13, Jian Lu13, Jing-Jia Luo14, Jiafu Mao15, Matthew S. Mizielinski2, Ryo Mizuta16, Paulo Nobre17, Masaki 18 4 19 20 10 Satoh , Enrico Scoccimarro , Tido Semmler , Justin Small , Jin-Song von Storch21 1Royal Netherlands Meteorological Institute, De Bilt, The Netherlands 2Met Office Hadley Centre, Exeter, UK 15 3University of Reading, Reading, UK 4 Centro Euro-Mediterraneo per i Cambiamenti Climatici, Bologna, Italy 5LASG, Institute of Atmospheric Physics, Chinese Academy of Sciences, Beijing , China P.R. 6Texas A&M University, College Station, Texas, USA 7National Research Council – Institute of Atmospheric Sciences and Climate, Italy 20 8Barcelona Super Computer Center, Earth Sciences Department, Barcelona, Spain 9Netherlands eScience Center, Amsterdam, The Netherlands 10Wageningen University, The Netherlands 11Japan Agency for Marine-Earth Science and Technology, Japan 12Swedish Meteorological and Hydrological Institute, Norrköping, Sweden 25 13Pacific Northwest National Laboratory, Richland, USA 14Bureau of Meteorology, Australia 15Environmental Sciences Division and Climate Change Science Institute, Oak Ridge National Laboratory, Oak Ridge, TN, USA 16Meteorological Research Institute,