Axonal Amyloid Precursor Protein and Its Fragments Undergo Somatodendritic Endocytosis and Processing
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Characterization of Efferosome Maturation and the Processing of Apoptotic Bodies
Western University Scholarship@Western Electronic Thesis and Dissertation Repository 8-15-2014 12:00 AM Characterization of Efferosome Maturation and the Processing of Apoptotic Bodies Yohan Kim The University of Western Ontario Supervisor Dr. Bryan Heit The University of Western Ontario Graduate Program in Microbiology and Immunology A thesis submitted in partial fulfillment of the equirr ements for the degree in Master of Science © Yohan Kim 2014 Follow this and additional works at: https://ir.lib.uwo.ca/etd Part of the Cell Biology Commons, Immunity Commons, and the Other Immunology and Infectious Disease Commons Recommended Citation Kim, Yohan, "Characterization of Efferosome Maturation and the Processing of Apoptotic Bodies" (2014). Electronic Thesis and Dissertation Repository. 2268. https://ir.lib.uwo.ca/etd/2268 This Dissertation/Thesis is brought to you for free and open access by Scholarship@Western. It has been accepted for inclusion in Electronic Thesis and Dissertation Repository by an authorized administrator of Scholarship@Western. For more information, please contact [email protected]. CHARACTERIZATION OF EFFEROSOME MATURATION AND THE PROCESSING OF APOPTOTIC BODIES (Thesis format: Monologue) by Yohan Kim Graduate Program in Microbiology and Immunology A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science The School of Graduate and Postdoctoral Studies The University of Western Ontario London, Ontario, Canada © Yohan Kim 2014 Abstract Every day billions of cells in our bodies undergo apoptosis and are cleared through efferocytosis – a phagocytosis-like process in which phagocytes engulf and degrade apoptotic cells. Proper processing of efferosomes prevents inflammation and immunogenic presentation of antigens. -

Transcytosis of Ngcam in Epithelial Cells Reflects Differential Signal
Published August 8, 2005 JCB: ARTICLE Transcytosis of NgCAM in epithelial cells reflects differential signal recognition on the endocytic and secretory pathways Eric Anderson, Sandra Maday, Jeff Sfakianos, Michael Hull, Bettina Winckler, David Sheff, Heike Fölsch, and Ira Mellman Department of Cell Biology, Ludwig Institute for Cancer Research, Yale University School of Medicine, New Haven, CT 06520 gCAM is a cell adhesion molecule that is largely ability to interact with clathrin adaptors. Based on the be- axonal in neurons and apical in epithelia. In Ma- havior of various NgCAM mutants, it appears that after Ndin-Darby canine kidney cells, NgCAM is tar- arrival at the basolateral surface, the AP-1B interaction geted to the apical surface by transcytosis, being first in- site is silenced by phosphorylation of Tyr33. This slows en- serted into the basolateral domain from which it is docytosis and inhibits basolateral recycling from endo- internalized and transported to the apical domain. Initial somes, resulting in NgCAM transcytosis due to a cryptic basolateral transport is mediated by a sequence motif apical targeting signal in its extracellular domain. Thus, Downloaded from (Y33RSL) decoded by the AP-1B clathrin adaptor complex. transcytosis of NgCAM and perhaps other membrane This motif is a substrate in vitro for tyrosine phosphoryla- proteins may reflect the spatial regulation of recognition tion by p60src, a modification that disrupts NgCAM’s by adaptors such as AP-1B. on March 10, 2017 Introduction The generation and maintenance of cellular polarity is an es- ously resorted in endosomes to maintain their polarized distri- sential aspect of multicellular life. -

Apical-To-Basolateral Transcytosis of Photoreceptor Outer Segments Induced by Lipid Peroxidation Products in Human Retinal Pigment Epithelial Cells
Retinal Cell Biology Apical-to-Basolateral Transcytosis of Photoreceptor Outer Segments Induced by Lipid Peroxidation Products in Human Retinal Pigment Epithelial Cells Tim U. Krohne,1 Frank G. Holz,1 and Ju¨rgen Kopitz2 PURPOSE. Progressive accumulation of extracellular material at posit formation and drusen biogenesis in AMD. (Invest Oph- the basolateral side of the retinal pigment epithelium (RPE) is thalmol Vis Sci. 2010;51:553–560) DOI:10.1167/iovs.09-3755 a key event in the pathogenesis of age-related macular degen- eration (AMD). The authors previously demonstrated that mod- rogressive deposition of extracellular material between the ifications with lipid peroxidation products, such as 4-hy- basolateral side of the retinal pigment epithelium (RPE) droxynonenal (HNE) and malondialdehyde (MDA), stabilize P and adjacent Bruch’s membrane is a hallmark of early-stage photoreceptor outer segment (POS) proteins against lysosomal age-related macular degeneration (AMD).1,2 Among the various degradation. Herein, they tested RPE cells for the basolateral histologically and clinically distinguishable manifestations of release of undegraded modified POS proteins. sub-RPE deposits, basal linear deposits (BLinD) and soft drusen METHODS. Polarized cultures of the human RPE cell line are recognized as specific for AMD.3,4 Both BLinD and soft ARPE-19 on permeable membranes were incubated with io- drusen represent accumulations of material described as mem- dine-125–labeled POS on the apical side. After 24 hours, radio- branous debris5 between the RPE basement membrane and the activity was quantified in apical medium, cell lysates, and inner collagenous layer of Bruch’s membrane and are, there- basolateral medium after separation of undegraded proteins by fore, considered diffuse and focal manifestations, respectively, precipitation. -

Slow Axonal Transport and Presynaptic Targeting of Clathrin Packets
bioRxiv preprint doi: https://doi.org/10.1101/2020.02.20.958140; this version posted February 20, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Slow Axonal Transport and Presynaptic Targeting of Clathrin Packets Archan Ganguly 1,2, Florian Wernert3, Sébastien Phan 2,4, Daniela Boassa 2,4, Utpal Das 1,2, Rohan Sharma 1,2, Ghislaine Caillol 3, Xuemei Han 5, John R. Yates III 5, Mark H. Ellisman 2,4, Christophe Leterrier 3, and Subhojit Roy 1,2 * 1 Department of Pathology, University of California, San Diego, La Jolla, CA 2 Department of Neurosciences, University of California, San Diego, La Jolla, CA 3 Aix Marseille Université, CNRS, INP UMR7051, NeuroCyto, Marseille, France 4 National Center for Microscopy and Imaging Research, University of California, San Diego, La Jolla, CA 5 Department of Cell Biology, The Scripps Research Institute, La Jolla, CA * Correspondence: [email protected] SUMMARY Clathrin has established roles in endocytosis, with clathrin-cages enclosing membrane infoldings, followed by rapid disassembly and reuse of clathrin-monomers. In neurons, clathrin synthesized in cell- bodies is conveyed via slow axonal transport – enriching at presynapses – however, mechanisms underlying transport/targeting are unknown, and its role at synapses is debated. Combining live- imaging, mass-spectrometry (MS), Apex-labeled EM-tomography and super-resolution, we found that unlike dendrites where clathrin assembles and disassembles as expected, axons contain stable ‘clathrin transport-packets’ that move intermittently with an anterograde bias, conveying endocytosis-related proteins. -

Thyroid Hormone Synthesis Continues Despite Biallelic Thyroglobulin Mutation with Cell Death
Thyroid hormone synthesis continues despite biallelic thyroglobulin mutation with cell death Xiaohan Zhang, … , Viviana A. Balbi, Peter Arvan JCI Insight. 2021. https://doi.org/10.1172/jci.insight.148496. Research In-Press Preview Endocrinology Graphical abstract Find the latest version: https://jci.me/148496/pdf Thyroid hormone synthesis continues despite biallelic thyroglobulin mutation with cell death Authors: Xiaohan Zhang1, Aaron P. Kellogg1, Cintia E. Citterio1,2,3, Hao Zhang1, Dennis Larkin1, Yoshiaki Morishita1,4, Héctor M. Targovnik2,3, Viviana Balbi5, and Peter Arvan1* Affiliations: 1Division of Metabolism, Endocrinology & Diabetes, University of Michigan, Ann Arbor, MI 48105 2Universidad de Buenos Aires. Facultad de Farmacia y Bioquímica. Departamento de Microbiología, Inmunología, Biotecnología y Genética/Cátedra de Genética. Buenos Aires, Argentina. 3CONICET, Universidad de Buenos Aires, Instituto de Inmunología, Genética y Metabolismo (INIGEM), Buenos Aires, Argentina 4Division of Diabetes, Department of Internal Medicine, Aichi Medical University, 1-1 Yazakokarimata, Nagakute, Aichi 480-1195, Japan 5Department of Endocrinology and Growth, Hospital de Niños Sor María Ludovica, La Plata, Argentina Conflict of interest: The authors declare that no conflict of interest exists. Short Title: Thyroxine synthesis from dead cells 1 Complete absence of thyroid hormone is incompatible with life in vertebrates. Thyroxine is synthesized within thyroid follicles upon iodination of thyroglobulin conveyed from the endoplasmic reticulum (ER), via the Golgi complex, to the extracellular follicular lumen. In congenital hypothyroidism from bi-allelic thyroglobulin mutation, thyroglobulin is misfolded and cannot advance from the ER, eliminating its secretion and triggering ER stress. Nevertheless, untreated patients somehow continue to synthesize sufficient thyroxine to yield measurable serum levels that sustain life. -

Axonal Transport and the Eye
Br J Ophthalmol: first published as 10.1136/bjo.60.8.547 on 1 August 1976. Downloaded from Brit. 7. Ophthal. (1976) 60, 547 Editorial: Axonal transport and the eye Since the earliest days of histology, it has been and proteins, and are predominantly found in the known that the neurons have a large amount of particulate fraction of nerve homogenates. The basophilic material in the cell body. More recently slower transported material is mainly soluble this has been shown to be due to masses of rough protein (McEwen and Grafstein, I968; Kidwai and endoplasmic reticulum which are responsible for a Ochs, I969; Sj6strand, 1970). Much of the former high rate of protein synthesis of neurons. Since the and more than 6o per cent of the latter is probably earliest days of the microscopy of living cells in neurotubular protein (tubulin), and the neurotubules vitro, particulate movement within these cells has are thought to play a major part in axonal transport been recognized, particularly in neurons (Nakai, (Schmitt and Samson, I968). The neurotubules I964). The last 30 years have seen the gradual have a similar structure to actin, and are linked to accumulation of the knowledge required to explain high-energy phosphate compounds. Myosin-like these two observations. In the last io years in proteins are also found in axoplasm. It is suggested particular, the development of radioactive tracer that moving material might in part roll along the techniques has revealed much information on the neurotubules, or that the neurotubules themselves movement of protein and other substances within might move by a mechanism involving myosin- axons, a process termed axonal transport (Barondes, guanosine triphosphate-neurotubule cross bonds I967). -

Vesicular Glycolysis Provides On-Board Energy for Fast Axonal Transport
Vesicular Glycolysis Provides On-Board Energy for Fast Axonal Transport Diana Zala,1,2,3 Maria-Victoria Hinckelmann,1,2,3 Hua Yu,1,2,3 Marcel Menezes Lyra da Cunha,1,4 Ge´ raldine Liot,1,2,3,6 Fabrice P. Cordelie` res,1,5 Sergio Marco,1,4 and Fre´ de´ ric Saudou1,2,3,* 1Institut Curie, 91405 Orsay, France 2CNRS UMR3306, 91405 Orsay, France 3INSERM U1005, 91405 Orsay, France 4INSERM U759, 91405 Orsay, France 5IBiSA Cell and Tissue Imaging Facility, Institut Curie, 91405 Orsay, France 6Present address: Institut Curie, CNRS UMR 3347, INSERM U1021, Universite´ Paris-Sud 11, 91405 Orsay, France *Correspondence: [email protected] http://dx.doi.org/10.1016/j.cell.2012.12.029 SUMMARY and Vale, 2009), nerve cells must have energy sources distrib- uted throughout their axons for these molecular motors to Fast axonal transport (FAT) requires consistent function. energy over long distances to fuel the molecular Glucose is the major source of brain energy and is required for motors that transport vesicles. We demonstrate normal brain function. Glucose breakdown occurs in the cytosol that glycolysis provides ATP for the FAT of vesicles. through the glycolytic pathway that converts glucose into pyru- Although inhibiting ATP production from mitochon- vate and generates free energy (Ames, 2000). The net energetic dria did not affect vesicles motility, pharmacological balance of glycolysis is relatively low, with two ATP being produced from one molecule of glucose. In contrast to glycol- or genetic inhibition of the glycolytic enzyme GAPDH ysis, mitochondria produce 34 ATP molecules (Ames, 2000) reduced transport in cultured neurons and in and provide most of the energy used by the brain. -

Transcytosis of Trka Leads to Diversification of Dendritic Signaling
www.nature.com/scientificreports OPEN Transcytosis of TrkA leads to diversifcation of dendritic signaling endosomes Received: 8 January 2018 Kelly Barford 1, Austin Keeler2, Lloyd McMahon1, Kathryn McDaniel1, Chan Choo Yap1, Accepted: 5 March 2018 Christopher D. Deppmann2,3 & Bettina Winckler1 Published: xx xx xxxx The development of the peripheral nervous system relies on long-distance signaling from target organs back to the soma. In sympathetic neurons, this long-distance signaling is mediated by target derived Nerve Growth Factor (NGF) interacting with its axonal receptor, TrkA. This ligand receptor complex internalizes into what is commonly referred to as the signaling endosome which is transported retrogradely to the soma and dendrites to mediate survival signaling and synapse formation, respectively. The molecular identity of signaling endosomes in dendrites has not yet been determined. Here, we perform a detailed analysis of TrkA endosomal compartments and trafcking patterns. We fnd that signaling endosomes are not uniform but molecularly diversifed into Rab7 (late endosome) and Rab11 (recycling endosome) populations in axons and dendrites in vitro and in the soma in vivo. Surprisingly, TrkA-NGF signaling endosomes in dendrites undergo dynamic trafcking events, including putative fusion and fssion. Overall, we fnd that signaling endosomes do not remain as a singular endosomal subtype but instead exist in multiple populations that undergo dynamic endosomal trafcking events. These dynamic events might drive functional diversifcation of the signaling endosome. Te development of the sympathetic and sensory nervous systems requires the target derived neurotrophic factor, Nerve Growth Factor (NGF)1. Te molecular and cellular mechanisms that enable target derived NGF to transmit signals long distances from distal axons to the cell body have been under intense investigation for several years. -

A New Look at Transudation: the Apocrine Connection
Physiol. Res. 69: 227-244, 2020 https://doi.org/10.33549/physiolres.934229 REVIEW A New Look at Transudation: The Apocrine Connection Robert FARKAŠ1, Milan BEŇO1, Denisa BEŇOVÁ-LISZEKOVÁ1, Ivan RAŠKA2, Otakar RAŠKA2,3 1Laboratory of Developmental Genetics, Institute of Experimental Endocrinology, Biomedical Research Center, Slovak Academy of Sciences, Bratislava, Slovak Republic, 2Institute of Biology and Medical Genetics, First Faculty of Medicine, Charles University, Prague, Czech Republic, 3Institute of Pathophysiology, Third Faculty of Medicine, Charles University, Prague, Czech Republic Received June 9, 2019 Accepted December 20, 2019 Epub Ahead of Print March 23, 2020 Summary Corresponding authors Transcellular trafficking in which various molecules are Robert Farkaš, Laboratory of Developmental Genetics, Institute transported across the interior of a cell, is commonly classified as of Experimental Endocrinology, Biomedical Research Center, transcytosis. However, historically this term has been used Slovak Academy of Sciences, Dúbravská cesta 9, 84505 synonymously with transudation. In both cases transcellular Bratislava, Slovak Republic. E-mail: [email protected]. Otakar trafficking starts with the internalization of proteins or other Raška, Third Faculty of Medicine, Charles University, Ruská 87, compounds on the basal or basolateral side of a cell and 10000 Prague, Czech Republic. E-mail: [email protected] continues by their transport across the interior to the apical pole (or vice versa) where they are subsequently released. -

Epithelial Cells Inhibit HIV-1 Transcytosis Across Human HIV-1
Mucosal and Plasma IgA from HIV-1-Exposed Uninfected Individuals Inhibit HIV-1 Transcytosis Across Human Epithelial Cells This information is current as of September 30, 2021. Claudia Devito, Kristina Broliden, Rupert Kaul, Lennart Svensson, Kari Johansen, Peter Kiama, Joshua Kimani, Lucia Lopalco, Stefania Piconi, Job J. Bwayo, Francis Plummer, Mario Clerici and Jorma Hinkula J Immunol 2000; 165:5170-5176; ; Downloaded from doi: 10.4049/jimmunol.165.9.5170 http://www.jimmunol.org/content/165/9/5170 http://www.jimmunol.org/ References This article cites 39 articles, 10 of which you can access for free at: http://www.jimmunol.org/content/165/9/5170.full#ref-list-1 Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists by guest on September 30, 2021 • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2000 by The American Association of Immunologists All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. Mucosal and Plasma IgA from HIV-1-Exposed Uninfected Individuals Inhibit HIV-1 Transcytosis Across Human Epithelial Cells1 Claudia Devito,* Kristina Broliden,2* Rupert Kaul,† Lennart Svensson,‡ Kari Johansen,‡ Peter Kiama,† Joshua Kimani,† Lucia Lopalco,§ Stefania Piconi,¶ Job J. -

Endocytic Deficiency Induced by ITSN-1S Knockdown Alters the Smad2/3-Erk1/2 Signaling Balance Downstream of Alk5
ß 2015. Published by The Company of Biologists Ltd | Journal of Cell Science (2015) 128, 1528–1541 doi:10.1242/jcs.163030 RESEARCH ARTICLE Endocytic deficiency induced by ITSN-1s knockdown alters the Smad2/3-Erk1/2 signaling balance downstream of Alk5 Cristina Bardita1, Dan N. Predescu1,2, Fei Sha1, Monal Patel1, Ganesh Balaji3 and Sanda A. Predescu1,2,* ABSTRACT leading to pulmonary edema, evidence suggests that apoptosis plays a beneficial role during ALI resolution owing to the pro- Recently, we demonstrated in cultured endothelial cells and in vivo regenerative role of clearance of apoptotic cells (Predescu et al., that deficiency of an isoform of intersectin-1, ITSN-1s, impairs 2013; Schmidt and Tuder, 2010). This effect is mediated through caveolae and clathrin-mediated endocytosis and functionally the production of growth factors including TGFb by macrophages upregulates compensatory pathways and their morphological engulfing apoptotic cells, or perhaps by other vascular cells carriers (i.e. enlarged endocytic structures, membranous rings or (Bardita et al., 2013; Henson and Tuder, 2008). TGFb, owing to tubules) that are normally underrepresented. We now show that its anti-inflammatory properties confines the extent of septal these endocytic structures internalize the broadly expressed injury and speeds recovery in ALI (D’Alessio et al., 2009). transforming growth factor b receptor I (TGFb-RI or TGFBR1), We have recently shown that in vivo deficiency of ITSN-1s, an also known as Alk5, leading to its ubiquitylation and degradation. isoform of ITSN-1 that is highly prevalent in lung endothelium and Moreover, the apoptotic or activated vascular cells of the ITSN-1s- deficiency of which is relevant to the pathology of ALI/ARDS knockdown mice release Alk5-bearing microparticles to the (Bardita et al., 2013; Predescu et al., 2013), induces extensive lung systemic circulation. -
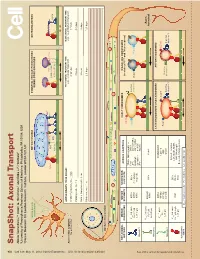
Snapshot: Axonal Transport Alison Twelvetrees,1,2 Adam G
950 Cell SnapShot: Axonal Transport 149 Alison Twelvetrees,1,2 Adam G. Hendricks,1 and Erika L.F. Holzbaur1 , May11, 2012©2012Elsevier Inc. DOI 10.1016/j.cell.2012.05.001 1University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104, USA 2Cancer Research UK London Research Institute, London WC2A 3LY, UK Mixed polarity MITOCHONDRIA SYNAPTIC VESICLE PRECURSORS NEUROFILAMENTS microtubules DENSE CORE GRANULES Syntabulin Miro FEZ1 Rab3 Liprin-α Dynein TRAKs JIP1 KBP DENN/MADD KIF5 KIF5A KIF1Bα KIF1A/KIF1Bβ MICROTUBULE AXONS DRAWN TO 5X SCALE: FAST AXONAL TRANSPORT TIME SLOW AXONAL TRANSPORT TIME Axon initial segment e.g., vesicular transport e.g., neurolament transport Selective lter Inhibitory interneuron - 1 mm 4 minutes 3 hours Purkinje cell - 36 mm 2 hours 4.5 days Retinal Ganglion cell - 5 cm 3 hours 6 days Motor neuron - 1 m 2.5 days 125 days Axonal cross-section + Uniform polarity microtubules + + KEY AXONAL MOTOR MOTOR NONMOTOR AXONAL ADAPTORS EARLY ENDOSOMES SIGNALING ENDOSOMES MOTORS PROPERTIES SUBUNITS SUBUNITS (contain p75NTR and Trk receptors) EEA1 TRAKs (Milton) and Miro V 0.8 µm/s KIF5A KLCs Fez1 DISC1 Inactive max JIPs Slp1/CRMP-2 Rab5 KIF5B (not always kinesin-2 Rab7 Rab5 Fs 5-7 pN Huntingtin LIS1/NUDEL See online version for legend and references. Lr 1-2 µm KIF5C required) Syntabulin mNUDC Kinesin-1 APP HSc70 V 0.43 µm/s Axon max KIF3A/B KAP3 Fodrin Fs 5 pN KIF3C terminal Lr 0.45 µm Kinesin-2 LATE ENDOSOMES AND LYSOSOMES AUTOPHAGOSOMES KIF1A DENN/MADD V 1 µm/s max KIF1Bα Liprin-α LAMP1 LC3-II Fs - KIF1Bβ KBP Rab7 Lr 1 µm Inactive Kinesin-3 KIF13B PIP3BP Inactive kinesin-2 Inactive kinesin-1 kinesin-1 Dynactin complex V 0.8 µm/s DICs max LIS1, NudE, NuDEL Fs 1 pN, 6 pN DHC DLICs HAP1/Huntingtin Lr 1 µm DLCs Bicaudal-D family proteins Cytoplasmic dynein Vmax= Maximal velocity Fs = Stall force Lr = Length of run SnapShot: Axonal Transport Alison Twelvetrees,1,2 Adam G.