Axonal Transport and the Eye
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Brain-Specific Expression of MAP2 Detected Using a Cloned Cdna Probe Sally A
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by PubMed Central Brain-specific Expression of MAP2 Detected Using a Cloned cDNA Probe Sally A. Lewis,* Alfredo Villasante,* Peter Sherline,* and Nicholas J. Cowan* *Department of Biochemistry, New York University Medical Center, New York, New York 10016; *Division of Endocrinology, Cornell University Medical College, New York, New York 10021 Abstract. We describe the isolation of a set of over- consistent with the coding capacity required by a lapping cDNAs encoding mouse microtubule associ- 250,000-D protein. ated protein 2 (MAP2), using an anti-MAP antiserum The MAP2-specific cloned cDNA probes were used to screen a mouse brain cDNA expression library in RNA blot transfer experiments to assay for the cloned in bacteriophage hgt 11. The authenticity of presence of MAP2 mRNA in a variety of mouse tis- these clones was established by the following criteria: sues. Though brain contained abundant quantities of (a) three non-identical clones each expressing a MAP2 mRNA, no corresponding sequences were de- MAP2 immunoreactive fusion protein were independ- tectable in RNA prepared from liver, kidney, spleen, ently isolated from the expression library; each of stomach, or thymus. We conclude that the expression these clones cross-hybridized at the nucleic acid level; of MAP2 is brain-specific. (b) anti-MAP antiserum was affinity purified using Use of the MAP2 specific cDNA probes in genomic nitrocellulose-bound fusion protein; these antibodies Southern blot transfer experiments showed the pres- detected only MAP2 in an immunoblot experiment of ence of a single gene encoding MAP2 in mouse. -

Immunolocalization and Molecular Properties of a High Molecular Weight Microtubule-Bundling Protein (Syncolin) from Chicken Erythrocytes E Feick, R
Immunolocalization and Molecular Properties of a High Molecular Weight Microtubule-bundling Protein (Syncolin) from Chicken Erythrocytes E Feick, R. Foisner, and G. Wiche Institute of Biochemistry, University of Vienna, 1090 Vienna, Austria Abstract. A protein of apparent molecular weight was displaced from the polymer by salt. Brain as well 280,000 (syncolin), which is immunoreactive with an- as erythrocyte microtubules, reconstituted with taxol tibodies to hog brain microtubule-associated protein from MAP-free tubulin and purified syncolin, were ag- (MAP) 2, was purified from chicken erythrocytes. Im- gregated into dense bundles containing up to 15 Downloaded from http://rupress.org/jcb/article-pdf/112/4/689/1060909/689.pdf by guest on 29 September 2021 munofluorescence microscopy of bone marrow cells microtubules, as determinedby'etectron microscopy. revealed the presence of syncolin in cells at all stages On the ultrastructural level, syncolin molecules were of erythrocyte differentiation. In early erythroblasts visualized as globular or ringlike structures, in con- syncolin was diffusely distributed throughout the trast to the thin, threadlike appearance of filamentous cytoplasm. At later stages it was found along microtu- MAPs, such as brain MAP 2. According to ultrastruc- bules of the marginal band, as confirmed by immuno- tural measurements and gel permeation chromatogra- electron microscopy. The association of syncolin with phy, syncolin's molecular weight was ,',,1 x 106. It is the marginal band was dependent on the integrity of suggested that syncolin's specific function is the cross- microtubules, as demonstrated by temperature- linking of microtubules in the marginal band and, by dependent de- and repolymerization or marginal band implication, the stabilization of this structure typical microtubules. -

And High-Molecular-Weight Forms of MAP2 in the Developing Cerebellum
The Journal of Neuroscience, December 1999, 8(12): 4503-4512 The Sequential Appearance of Low- and High-Molecular-Weight Forms of MAP2 in the Developing Cerebellum R. P. Tucker,’ L. I. Binder,’ C. Viereck,’ B. A. Hemmings,’ and A. I. Matus’ ‘Friedrich Miescher Institute, CH-4002 Basel, Switzerland, and *Department of Cell Biology and Anatomy, School of Medicine and Dentistry, University of Alabama at Birmingham, Birmingham, Alabama 35294 Mammalian microtubule-associated protein 2 (MAPS) exists shown that a number of MAPS promote the assemblyof tubulin in high-molecular-weight (M, -280,000) and low-molecular- into microtubulesand stabilize the resulting microtubulesagainst weight (M, -70,000) forms, with the latter protein being more the depolymerizing effects of cold and various pharmacological abundant in embryonic brain homogenates than in prepa- agents(Olmsted, 1986; Matus, 1988). The most abundant MAP rations from mature brain (Riederer and Matus, 1985). In the in the mammalian CNS, MAP2, displays each of these char- current study, we have shown that avian MAP2 also exists acteristics: (1) There are 2 closely related high-molecular-weight as both high- (M, -260,000) and low-molecular-weight (M, (HMW) forms of MAP2, MAP2a, and MAP2b, which change -65,000) forms whose relative abundance changes during in relative abundanceduring development (Binder et al., 1984; brain maturation, indicating a conserved function for these Burgoyne and Cummings, 1984);(2) MAP2 is found in dendrites proteins during vertebrate neuronal morphogenesis. Using and only rarely in axons (e.g., Cacereset al., 1984; DeCamilli indirect immunohistochemistry, we have determined the cel- et al., 1984; Huber and Matus, 1984b); and (3) MAP2 stabilizes lular distribution of the high- and low-molecular-weight forms and promotes the assembly of microtubules in vitro (Murphy of MAP2 in the developing avian cerebellum. -

Evidence for Conformational Change-Induced Hydrolysis of Β-Tubulin-GTP
bioRxiv preprint doi: https://doi.org/10.1101/2020.09.08.288019; this version posted September 10, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Evidence for conformational change-induced hydrolysis of β-tubulin-GTP Mohammadjavad Paydar1 and Benjamin H. Kwok1† 1 Institute for Research in Immunology and Cancer (IRIC), Département de médecine, Université de Montréal, P.O. Box 6128, Station Centre-Ville, Montréal, QC H3C 3J7, Canada. † Corresponding author: B. H. Kwok: [email protected] Keywords: Kinesin, Kif2A, MCAK, motor protein, microtubules, tubulin, GTP hydrolysis Abbreviations NM: Neck+Motor MD: Motor domain MT: Microtubule Running title: Tubulin GTP hydrolysis 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.09.08.288019; this version posted September 10, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. ABSTRACT Microtubules, protein polymers of α/β-tubulin dimers, form the structural framework for many essential cellular processes including cell shape formation, intracellular transport, and segregation of chromosomes during cell division. It is known that tubulin-GTP hydrolysis is closely associated with microtubule polymerization dynamics. However, the precise roles of GTP hydrolysis in tubulin polymerization and microtubule depolymerization, and how it is initiated are still not clearly defined. We report here that tubulin-GTP hydrolysis can be triggered by conformational change induced by the depolymerizing kinesin-13 proteins or by the stabilizing chemical agent paclitaxel. We provide biochemical evidence that conformational change precedes tubulin-GTP hydrolysis, confirming this process is mechanically driven and structurally directional. -

Slow Axonal Transport and Presynaptic Targeting of Clathrin Packets
bioRxiv preprint doi: https://doi.org/10.1101/2020.02.20.958140; this version posted February 20, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Slow Axonal Transport and Presynaptic Targeting of Clathrin Packets Archan Ganguly 1,2, Florian Wernert3, Sébastien Phan 2,4, Daniela Boassa 2,4, Utpal Das 1,2, Rohan Sharma 1,2, Ghislaine Caillol 3, Xuemei Han 5, John R. Yates III 5, Mark H. Ellisman 2,4, Christophe Leterrier 3, and Subhojit Roy 1,2 * 1 Department of Pathology, University of California, San Diego, La Jolla, CA 2 Department of Neurosciences, University of California, San Diego, La Jolla, CA 3 Aix Marseille Université, CNRS, INP UMR7051, NeuroCyto, Marseille, France 4 National Center for Microscopy and Imaging Research, University of California, San Diego, La Jolla, CA 5 Department of Cell Biology, The Scripps Research Institute, La Jolla, CA * Correspondence: [email protected] SUMMARY Clathrin has established roles in endocytosis, with clathrin-cages enclosing membrane infoldings, followed by rapid disassembly and reuse of clathrin-monomers. In neurons, clathrin synthesized in cell- bodies is conveyed via slow axonal transport – enriching at presynapses – however, mechanisms underlying transport/targeting are unknown, and its role at synapses is debated. Combining live- imaging, mass-spectrometry (MS), Apex-labeled EM-tomography and super-resolution, we found that unlike dendrites where clathrin assembles and disassembles as expected, axons contain stable ‘clathrin transport-packets’ that move intermittently with an anterograde bias, conveying endocytosis-related proteins. -

Tubulin Requires Tau for Growth Onto Microtubule Initiating Sites (Flagella/In Vitro Assembly/Electron Microscopy) GEORGE B
Reprinted from Proc. Natl. Acad. Sci. USA Vol. 73, No. 11, pp. 4070-4074, November 1976 Cell Biology Tubulin requires tau for growth onto microtubule initiating sites (flagella/in vitro assembly/electron microscopy) GEORGE B. WITMAN, DON W. CLEVELAND, MURRAY D. WEINGARTEN, AND MARC W. KIRSCHNER The Departments of Biology and Biochemistry, Princeton University, Princeton, New Jersey 08540 Communicated by J. T. Bonner, August 9, 1976 ABSTRACT Tubulin purified by phosphocellulose chro- ether)-N,N'-tetraacetic acid (EGTA), 1 mM GTP, 0.5 mM matography and free of accessory proteins will not form mi- MgCl2, and 1 mM mercaptoethanol]. A high-speed supernatant crotubules in the absence or presence of micrdtubule initiating sites (flagellar microtubules). The capacity for growth onto of depolymerized microtubules was prepared by cooling the pre-existing "seeds" can be restored by the addition of small protein at 5 mg/ml in the above buffer at 0° for 30 min and quantities of partially purified tau protein. Larger quantities sedimenting the solution at 190,000 X g for 2 hr. Phosphocel- restore the capacity for spontaneous assembly. These results lulose-purified tubulin was prepared as described (4, 8). Mi- suggest that tubulin requires tau for both initiation and growth crotubule protein in purification buffer was repolymerized and of microtubules and that tau is incorporated into the microtu- centrifuged at 100,000 X g for 40 min at 280. The resulting bule throughout its length. pellets were resuspended in Mes-EDTA buffer (25 mM Mes, pH 6.4,0.5 mM MgCl2, 0.1 mM EDTA, and 1 mM mercapto- The development of procedures for the assembly of microtu- ethanol) to a final concentration of 8-10 mg/ml. -

Axon Growth: Roles of Microfilaments and Microtubules* Kenneth M
Proceedings of the JVational Academy of Sciences Vol. 66, No. 4, pp. 1206-1212, August 1970 Axon Growth: Roles of Microfilaments and Microtubules* Kenneth M. Yamada, Brian S. Spooner, and Norman K. Wessellst DEPARTMENT OF BIOLOGICAL SCIENCES, STANFORD UNIVERSITY, STANFORD, CALIFORNIA Communicated by Colin S. Pittendrigh, April 27, 1970 Abstract. The motile tips of elongating axons consist of growth cones from which microspikes protrude. Cytochalasin B causes retraction of microspikes, rounding-up of growth cones, and cessation of axon elongation. Drug with- drawal is followed by resumption of growth cone and microspike activity and of axon elongation. In contrast, colchicine causes shortening and retraction of axons, but it does not initially affect the tips. Growth cones and microspikes of elongating axons contain a network of 50 A microfilaments, the pattern of which is altered by cytochalasin treatment. These experiments indicate that both structural integrity of the axon and continuing function of its motile tip are essential elements in axonal elongation. The tips of elongating axons consist of expanded regions called "growth cones" from which project long slender microspikes (filopodia). These microspikes continually wave about, extend, and retract as the growth cone moves over a substratum.1 Although it is assumed that growth cones and microspikes play a significant role in axon extension, it has not been previously possible to analyze their functions. We have investigated the effects of cytochalasin B on axon elongation since this drug halts cell movement,2 a process similar in many respects to growth cone movement. Cytochalasin B is also known to inhibit cytokinesis3 and morpho- genetic movements,4 apparently by disrupting contractile 50 A microfilaments. -

Vesicular Glycolysis Provides On-Board Energy for Fast Axonal Transport
Vesicular Glycolysis Provides On-Board Energy for Fast Axonal Transport Diana Zala,1,2,3 Maria-Victoria Hinckelmann,1,2,3 Hua Yu,1,2,3 Marcel Menezes Lyra da Cunha,1,4 Ge´ raldine Liot,1,2,3,6 Fabrice P. Cordelie` res,1,5 Sergio Marco,1,4 and Fre´ de´ ric Saudou1,2,3,* 1Institut Curie, 91405 Orsay, France 2CNRS UMR3306, 91405 Orsay, France 3INSERM U1005, 91405 Orsay, France 4INSERM U759, 91405 Orsay, France 5IBiSA Cell and Tissue Imaging Facility, Institut Curie, 91405 Orsay, France 6Present address: Institut Curie, CNRS UMR 3347, INSERM U1021, Universite´ Paris-Sud 11, 91405 Orsay, France *Correspondence: [email protected] http://dx.doi.org/10.1016/j.cell.2012.12.029 SUMMARY and Vale, 2009), nerve cells must have energy sources distrib- uted throughout their axons for these molecular motors to Fast axonal transport (FAT) requires consistent function. energy over long distances to fuel the molecular Glucose is the major source of brain energy and is required for motors that transport vesicles. We demonstrate normal brain function. Glucose breakdown occurs in the cytosol that glycolysis provides ATP for the FAT of vesicles. through the glycolytic pathway that converts glucose into pyru- Although inhibiting ATP production from mitochon- vate and generates free energy (Ames, 2000). The net energetic dria did not affect vesicles motility, pharmacological balance of glycolysis is relatively low, with two ATP being produced from one molecule of glucose. In contrast to glycol- or genetic inhibition of the glycolytic enzyme GAPDH ysis, mitochondria produce 34 ATP molecules (Ames, 2000) reduced transport in cultured neurons and in and provide most of the energy used by the brain. -

Neurons and Associated Cells
CH02.p3 3/11/05 8:41 AM Page 11 2 Neurons and Associated Cells Neuroplasticity Organelles and Components Neurotrophic Factors and Tropic Factors Structure of Peripheral Nerves and Ganglia Neuroglia (GLIA) Paraneurons Nerve Regeneration Plasticity and Axonal Sprouting resulting excitation rapidly to other portions of NEUROPLASTICITY the nerve cell, and to influence other neurons, muscle cells, and glandular cells. Neurons are Some 100–200 billion ([1–2] × 1011) neu- so specialized that most are incapable of repro- rons (nerve cells), as well as many more glial ducing themselves and they lose viability if cells, are integrated into the structural and denied an oxygen supply for more than a few functional fabric that is the brain. They exhibit minutes. a wide diversity of form and sizes. The neuron is the basic unit of the nervous system and is composed of four structurally defined regions: a cell body (soma) that emits a single nerve ORGANELLES AND COMPONENTS process called an axon, which ends at presy- naptic terminals, and a variable number of Each neuron consists of a large nucleus, a branching processes called dendrites (Figs. 2.1 plasma (cell) membrane and cytoplasm con- and 2.2). Each axon, including its collateral sisting of cytosol (fluid phase of cytoplasm), branches, usually terminates as an arbor of fine and a number of organelles, including the fibers; each fiber ends as an enlargement called endoplasmic reticulum, Nissl substance, Golgi a bouton,which is part of a synaptic junction. apparatus, mitochondria, lysosomes and cyto- At the other end of the neuron, there is a three- skeleton (Figs. -
Cyclic AMP-Dependent Endogenous Phosphorylation of a Microtubule-Associated Protein (Protein Phosphorylation/In Vitro Assembly) ROGER D
Proc. Nat. Acad. Sci. USA Vol. 72, No. 1, pp. 177-181, January 1975 Cyclic AMP-Dependent Endogenous Phosphorylation of a Microtubule-Associated Protein (protein phosphorylation/in vitro assembly) ROGER D. SLOBODA, STEPHEN A. RUDOLPH, JOEL L. ROSENBAUM, AND PAUL GREENGARD Department of Biology, Kline Biology Tower, Yale University and Department of Pharmacology, Yale University School of Medicine, New Haven, Connecticut 06610 Communicated by Aaron B. Lerner, October 21, 1974 ABSTRACT Microtubules prepared from chick brain were homogenized in a motor-driven glass-Teflon homogenizer homogenates by successive cycles of assembly-disassembly at 00 in 1 ml of polymerization mixture buffer [1 mM MgSO4, were found to contain two high-molecular-weight pro- ether)-N,N'-tetra- teins, designated microtubule-associated protein, and 2 mM ethyleneglycol bis(#-aminoethyl microtubule-associated proteins. Microtubule-associated acetate (EGTA), 1 mM GTP, 100 mM piperazine-N,N'-bis(2- protein2 (apparent molecular weight 300,000 by sodium ethanesulfonic acid) (Pipes) (pH 6.9)] per g wet weight, and dodecyl sulfate-polyacrylamide gel electrophoresis) was the homogenate was centrifuged at 130,000 X g for 1 hr at 40 the preferred substrate for an endogenous cyclic AMP- to yield a crude high-speed supernatant. This supernatant was dependent protein kinase which appeared to be an integral component of the microtubules. The initial rate of phos- diluted with an equal volume of polymerization mixture buffer phorylation of microtubule-associated protein2 was en- containing 8 M glycerol, and incubated at 370 for 30 min to hanced 4- to 6-fold by cyclic AMP, with half-maximal assemble microtubules. -
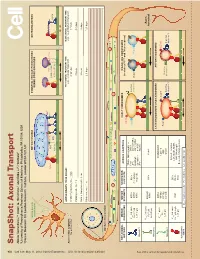
Snapshot: Axonal Transport Alison Twelvetrees,1,2 Adam G
950 Cell SnapShot: Axonal Transport 149 Alison Twelvetrees,1,2 Adam G. Hendricks,1 and Erika L.F. Holzbaur1 , May11, 2012©2012Elsevier Inc. DOI 10.1016/j.cell.2012.05.001 1University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104, USA 2Cancer Research UK London Research Institute, London WC2A 3LY, UK Mixed polarity MITOCHONDRIA SYNAPTIC VESICLE PRECURSORS NEUROFILAMENTS microtubules DENSE CORE GRANULES Syntabulin Miro FEZ1 Rab3 Liprin-α Dynein TRAKs JIP1 KBP DENN/MADD KIF5 KIF5A KIF1Bα KIF1A/KIF1Bβ MICROTUBULE AXONS DRAWN TO 5X SCALE: FAST AXONAL TRANSPORT TIME SLOW AXONAL TRANSPORT TIME Axon initial segment e.g., vesicular transport e.g., neurolament transport Selective lter Inhibitory interneuron - 1 mm 4 minutes 3 hours Purkinje cell - 36 mm 2 hours 4.5 days Retinal Ganglion cell - 5 cm 3 hours 6 days Motor neuron - 1 m 2.5 days 125 days Axonal cross-section + Uniform polarity microtubules + + KEY AXONAL MOTOR MOTOR NONMOTOR AXONAL ADAPTORS EARLY ENDOSOMES SIGNALING ENDOSOMES MOTORS PROPERTIES SUBUNITS SUBUNITS (contain p75NTR and Trk receptors) EEA1 TRAKs (Milton) and Miro V 0.8 µm/s KIF5A KLCs Fez1 DISC1 Inactive max JIPs Slp1/CRMP-2 Rab5 KIF5B (not always kinesin-2 Rab7 Rab5 Fs 5-7 pN Huntingtin LIS1/NUDEL See online version for legend and references. Lr 1-2 µm KIF5C required) Syntabulin mNUDC Kinesin-1 APP HSc70 V 0.43 µm/s Axon max KIF3A/B KAP3 Fodrin Fs 5 pN KIF3C terminal Lr 0.45 µm Kinesin-2 LATE ENDOSOMES AND LYSOSOMES AUTOPHAGOSOMES KIF1A DENN/MADD V 1 µm/s max KIF1Bα Liprin-α LAMP1 LC3-II Fs - KIF1Bβ KBP Rab7 Lr 1 µm Inactive Kinesin-3 KIF13B PIP3BP Inactive kinesin-2 Inactive kinesin-1 kinesin-1 Dynactin complex V 0.8 µm/s DICs max LIS1, NudE, NuDEL Fs 1 pN, 6 pN DHC DLICs HAP1/Huntingtin Lr 1 µm DLCs Bicaudal-D family proteins Cytoplasmic dynein Vmax= Maximal velocity Fs = Stall force Lr = Length of run SnapShot: Axonal Transport Alison Twelvetrees,1,2 Adam G. -

NERVE TISSUE Neuron – Nerve Cell
Department of Histology and Embryology, P. J. Šafárik University, Medical Faculty, Košice NERVE TISSUE: Sylabus for foreign students Author: doc. MVDr. Iveta Domoráková, PhD. Revised by: prof. MUDr. Eva Mechírová, CSc. NERVE TISSUE FUNCTION: Reception, transmission, processing of nerve stimuli. Coordination of all functional activities in the body: - motor function (body movement) - sensory (rapid response to external stimuli) - visceral, endocrine and exocrine glands - mental functions, memory, emotion A) Anatomically nervous system consists of: 1. CNS (central nervous system) – brain, spinal cord 2. PNS (peripheral nervous system) – peripheral nerves and ganglia B) Functionally nervous system is divided into the: 1. Somatic nervous system (sensory and motor innervation) 2. Autonomic nervous system (involuntary innervation of smooth muscles, glands) C) Microscopic structure of the nerve tissue - two types of cells: 1. Nerve cells – neurons 2. Glial cells (supporting, electrical insulation, metabolic function) Neuron – nerve cell - is the structural and functional unit of the nerve tissue - receives stimuli from other cells - conducts electrical impulses to another cells by their processes - chainlike communication - ten bilion of neurons in humans A. Neurons according the shape: Pyramidal (E) star-shaped (D) pear-shaped (G) oval (B) B. Types of neurons according number of the processes 1. multipolar (D,E,G)) 2. bipolar (A) 3. pseudounipolar (B) 4. unipolar C. Neurons - according the function Motor (efferent) neurons – convey impulses