GI Endocrine Function
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Calcium-Sensing Receptor Is a Physiologic Multimodal Chemosensor Regulating Gastric G-Cell Growth and Gastrin Secretion
Calcium-sensing receptor is a physiologic multimodal chemosensor regulating gastric G-cell growth and gastrin secretion Jianying Fenga, Clark D. Petersena, David H. Coyb, Jian-Kang Jiangc, Craig J. Thomasc, Martin R. Pollakd, and Stephen A. Wanka,1 aDigestive Diseases Branch, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD 20892-1804; bPeptide Research Laboratories, Department of Medicine, Tulane Health Sciences Center, New Orleans, LA 70112-2699; cNational Institutes of Health Chemical Genomics Center, National Human Genome Research Institute, National Institutes of Health, Bethesda, MD 20892; and dDivision of Nephrology, Beth Israel Deaconess Medical Center, Boston, MA 02215 Edited* by Martha Vaughan, National Heart, Lung, and Blood Institute, Bethesda, MD, and approved September 1, 2010 (received for review June 25, 2010) The calcium-sensing receptor (CaR) is the major sensor and The sensing of extracellular Ca2+ and the regulation of Ca2+ regulator of extracellular Ca2+, whose activity is allosterically reg- homestasis has been largely attributed to the calcium-sensing ulated by amino acids and pH. Recently, CaR has been identified in receptor (CaR), a member of the C family of G protein-coupled the stomach and intestinal tract, where it has been proposed to receptors (GPCR). Consistent with this function, CaR is ex- 2+ function in a non-Ca2+ homeostatic capacity. Luminal nutrients, pressed on extracellular Ca -regulating cells such as para- such as Ca2+ and amino acids, have been recognized for decades thyroid, thyroid parafollicular, renal tubular, and bone cells (6). as potent stimulants for gastrin and acid secretion, although the The CaR has a large NH2 terminal extracellular domain molecular basis for their recognition remains unknown. -

Parietal Cell Hyperplasia Induced by Long-Term Administration of Antacids to Rats
Gut: first published as 10.1136/gut.19.9.798 on 1 September 1978. Downloaded from Gut, 1978, 19, 798-801 Parietal cell hyperplasia induced by long-term administration of antacids to rats G. MAZZACCA1, F. CASCIONE, G. BUDILLON, L. D'AGOSTINO, L. CIMINO, AND C. FEMIANO From the Division of Gastroenterology, 2nd School ofMedicine, University ofNaples, and the Department ofPathology, Institute for Tumoral Diseases, Naples, Italv SUMMARY Suspension of magnesium and aluminum hydroxide (30-60 mEq/24 h) or a comparable volume of water was orally administered by gastric intubation to two groups of 20 male wistar rats each over 60 days. The antacid treatment led to a significant increase in the height (0-464 + 0-02 mm v. 0-318 ± 0 06) and in the volume (472 ± 32 mm3 v. 328 ± 45) of the fundic mucosa ofthe stomach, in the average count of parietal cells per unit area of the mucosa (32-37 ± 1 8 v. 22-3 + 1 6), and in the total parietal cell population of the stomach (53-6 + 3.5 x 106 v. 43-2 + 3.7 x 106). Furthermore fasting serum gastrin concentration was significantly higher in the antacid treated rats (81.2 + 7.4 pg/ml) than in control animals (56-9 ± 6-9 pg/ml). It is known that a feedback mechanism governs the The animals of group B received each time and by relationship between antral gastrin release and the same procedure a comparable volume of water. intraluminal gastric pH (Walsh et al., 1975). During the night each animal was housed in a Furthermore, gastrin exerts a trophic influence on separate cage and the antacid suspension was the the gastric mucosa with increase in the parietal cell only drinking fluid available to the rats of group A. -

Overview of Gastrointestinal Function
Overview of Gastrointestinal Function George N. DeMartino, Ph.D. Department of Physiology University of Texas Southwestern Medical Center Dallas, TX 75390 The gastrointestinal system Functions of the gastrointestinal system • Digestion • Absorption • Secretion • Motility • Immune surveillance and tolerance GI-OP-13 Histology of the GI tract Blood or Lumenal Serosal Side or Mucosal Side Structure of a villus Villus Lamina propria Movement of substances across the epithelial layer Tight junctions X Lumen Blood Apical membrane Basolateral membrane X X transcellular X X paracellular GI-OP-19 Histology of the GI tract Blood or Lumenal Serosal Side or Mucosal Side Motility in the gastrointestinal system Propulsion net movement by peristalsis Mixing for digestion and absorption Separation sphincters Storage decreased pressure GI-OP-42 Intercellular signaling in the gastrointestinal system • Neural • Hormonal • Paracrine GI-OP-10 Neural control of the GI system • Extrinsic nervous system autonomic central nervous system • Intrinsic (enteric) nervous system entirely with the GI system GI-OP-14 The extrinsic nervous system The intrinsic nervous system forms complete functional circuits Sensory neurons Interneurons Motor neurons (excitatory and inhibitory) Parasympathetic nerves regulate functions of the intrinsic nervous system Y Reflex control of gastrointestinal functions Vago-vagal Afferent reflex Salivary Glands Composition of Saliva O Proteins α−amylase lactoferrin lipase RNase lysozyme et al mucus O Electrolyte solution water Na+ , K + - HCO3 -

The Metabolic Serine Hydrolases and Their Functions in Mammalian Physiology and Disease Jonathan Z
REVIEW pubs.acs.org/CR The Metabolic Serine Hydrolases and Their Functions in Mammalian Physiology and Disease Jonathan Z. Long* and Benjamin F. Cravatt* The Skaggs Institute for Chemical Biology and Department of Chemical Physiology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, California 92037, United States CONTENTS 2.4. Other Phospholipases 6034 1. Introduction 6023 2.4.1. LIPG (Endothelial Lipase) 6034 2. Small-Molecule Hydrolases 6023 2.4.2. PLA1A (Phosphatidylserine-Specific 2.1. Intracellular Neutral Lipases 6023 PLA1) 6035 2.1.1. LIPE (Hormone-Sensitive Lipase) 6024 2.4.3. LIPH and LIPI (Phosphatidic Acid-Specific 2.1.2. PNPLA2 (Adipose Triglyceride Lipase) 6024 PLA1R and β) 6035 2.1.3. MGLL (Monoacylglycerol Lipase) 6025 2.4.4. PLB1 (Phospholipase B) 6035 2.1.4. DAGLA and DAGLB (Diacylglycerol Lipase 2.4.5. DDHD1 and DDHD2 (DDHD Domain R and β) 6026 Containing 1 and 2) 6035 2.1.5. CES3 (Carboxylesterase 3) 6026 2.4.6. ABHD4 (Alpha/Beta Hydrolase Domain 2.1.6. AADACL1 (Arylacetamide Deacetylase-like 1) 6026 Containing 4) 6036 2.1.7. ABHD6 (Alpha/Beta Hydrolase Domain 2.5. Small-Molecule Amidases 6036 Containing 6) 6027 2.5.1. FAAH and FAAH2 (Fatty Acid Amide 2.1.8. ABHD12 (Alpha/Beta Hydrolase Domain Hydrolase and FAAH2) 6036 Containing 12) 6027 2.5.2. AFMID (Arylformamidase) 6037 2.2. Extracellular Neutral Lipases 6027 2.6. Acyl-CoA Hydrolases 6037 2.2.1. PNLIP (Pancreatic Lipase) 6028 2.6.1. FASN (Fatty Acid Synthase) 6037 2.2.2. PNLIPRP1 and PNLIPR2 (Pancreatic 2.6.2. -

Cellular Localization of Gastric Inhibitory Polypeptide in the Duodenum and Jejunum
Gut: first published as 10.1136/gut.14.4.284 on 1 April 1973. Downloaded from Gut, 1973, 14, 284-288 Cellular localization of gastric inhibitory polypeptide in the duodenum and jejunum JULIA M. POLAK, S. R. BLOOM', MARION KUZIO, J. C. BROWN, AND A. G. E. PEARSE From the Department ofHistochemistry, Royal Postgraduate Medical School, Hammersmith Hospital, London, and the Department ofPhysiology, University ofBritish Columbia, Vancouver, Canada SUMMARY Indirect immunofluorescence studies using an antiserum to purified porcine gastric inhibitory polypeptide indicate, in the gastrointestinal tract of dog and man, that this polypeptide is present in cells situated predominantly in the mid-zone of the glands in the duodenum and, to a lesser extent, in the jejunum. Absolute correlation of the gastric inhibitory polypeptide cell with one or other of the known endocrine-like cells identified by electron microscopy awaits confirmation by electron immunocytochemistry. It is here identified as an endocrine polypeptide cell of the APUD series and, provisionally, as the D, cell. While the hormonal status of a given polypeptide depends ultimately on physiological experiments the present results strengthen the view that gastric inhibitory polypeptide is indeed a hormone. In 1969 Brown, Pederson, Jorpes, and Mutt described We report here the results of immunofluorescence an enterogastrone, extractable from porcine intestine, studies on the localization of GIP in human and http://gut.bmj.com/ which strongly inhibited gastric acid secretion. The canine intestine. purification of an apparently similar enterogastrone was reported in the same year by Lucien, Itoh, Sun, Material and Methods Meyer, Carlton, and Schally. The first of these poly- peptides, named gastric inhibitory polypeptide Operative samples of duodenal and jejunal mucosa (GIP) by Brown, Mutt, and Pederson (1970), was from seven human subjects were studied. -

Biogenesis of Lipid Bodies in Lobosphaera Incisa
Biogenesis of Lipid Bodies in Lobosphaera incisa Dissertation for the award of the degree “Doctor rerum naturalium” of the Georg-August-Universität Göttingen within the doctoral program GGNB Microbiology and Biochemistry of the Georg-August University School of Science (GAUSS) submitted by Heike Siegler from Münster Göttingen 2016 Members of the Thesis Committee Prof. Dr. Ivo Feußner Department for Plant Biochemistry, Albrecht-von-Haller Institute for Plant Sciences, University of Göttingen Prof. Dr. Volker Lipka Department of Plant Cell Biology, Albrecht-von-Haller Institute for Plant Sciences, University of Göttingen Prof. Dr. Thomas Friedl Department of Experimental Phycology and Culture Collection of Algae at the University of Göttingen, Albrecht-von-Haller Institute for Plant Sciences, University of Göttingen Members of the Examination Board Prof. Dr. Ivo Feußner (Referee) Department for Plant Biochemistry, Albrecht-von-Haller Institute for Plant Sciences, University of Göttingen Prof. Dr. Volker Lipka (2nd Referee) Department of Plant Cell Biology, Albrecht-von-Haller Institute for Plant Sciences, University of Göttingen Prof. Dr. Thomas Friedl Department of Experimental Phycology and Culture Collection of Algae at the University of Göttingen, Albrecht-von-Haller Institute for Plant Sciences, University of Göttingen Prof. Dr. Andrea Polle Department of Forest Botany and Tree Physiology, Büsgen Institute, University of Göttingen PD Dr. Thomas Teichmann Department of Plant Cell Biology, Albrecht-von-Haller Institute for Plant Sciences, University of Göttingen Dr. Martin Fulda Department for Plant Biochemistry, Albrecht-von-Haller Institute for Plant Sciences, University of Göttingen Date of oral examination: 30.05.2016 Affidavit I hereby declare that I wrote the present dissertation on my own and with no other sources and aids than quoted. -

Expression of Cell Membrane Complement Regulatory Glycoproteins Along the Normal and Diseased Human Gastrointestinal Tract
522 Gut 1998;42:522–529 Expression of cell membrane complement regulatory glycoproteins along the normal and diseased human gastrointestinal tract Gut: first published as 10.1136/gut.42.4.522 on 1 April 1998. Downloaded from A E Berstad, P Brandtzaeg Abstract tract. Indeed, activated complement has been Background/Aims—Uncontrolled comple- detected in lesions of inflammatory bowel ment activation may be of immunopatho- disease,2–4 coeliac disease,5 and recently also in logical importance in inflammatory Helicobacter pylori gastritis.6 diseases of the gastrointestinal tract. Ex- Certain complement activation products, pression of membrane bound factors that notably the C3b fragment and the C5b-7 com- regulate complement activation was there- plex, can on their own—that is, without associ- fore studied in situ. ated antibody—bind to any nearby cell Methods—Frozen tissue specimens were membrane.7 Therefore it is crucial that com- obtained from patients with Helicobacter plement activity is tightly regulated. Mam- pylori gastritis, coeliac disease, Crohn’s malian cells are protected from complement disease, or ulcerative colitis, and from induced damage by a family of cell membrane histologically normal controls. Sections complement regulatory glycoproteins that were examined by immunofluorescence downregulate activation of homologous com- with monoclonal antibodies to protectin plement on their cell surface.8 Protectin (CD59), decay accelerating factor (DAF), (CD59) is broadly distributed on cells of and membrane cofactor protein (MCP). haemopoietic and non-haemopoietic origin79; Results—Protectin and MCP were widely it inhibits the formation of terminal comple- expressed in normal and diseased mu- ment complex by preventing the binding of C9 cosae. -

WO 2015/048577 A2 April 2015 (02.04.2015) W P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2015/048577 A2 April 2015 (02.04.2015) W P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61K 48/00 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (21) International Application Number: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, PCT/US20 14/057905 DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, 26 September 2014 (26.09.2014) KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, (25) Filing Language: English PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, (26) Publication Language: English SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: 61/883,925 27 September 2013 (27.09.2013) US (84) Designated States (unless otherwise indicated, for every 61/898,043 31 October 2013 (3 1. 10.2013) US kind of regional protection available): ARIPO (BW, GH, GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, (71) Applicant: EDITAS MEDICINE, INC. -
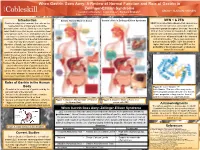
A Review of Normal Function and Role of Gastrin in Zollinger-Ellison
When Gastrin Goes Awry: A Review of Normal Function and Role of Gastrin in Zollinger-Ellison Syndrome Leigha LaTourette1, Janel Cross2, Barbara Brabetz2 Department of Plant and Animal Science1, Department of Natural Sciences and Mathematics2 Introduction Gastrin: Normal Mode of Action Gastrin’s Role in Zollinger-Ellison Syndrome MEN 1 & ZES Gastrin is a digestive hormone that acts on the MEN1 is an inheritable disease that causes over neuroendocrine and parietal cells of the secretion of hormones via tumors on the gastrointestinal tract to ultimately secrete gastric endocrine glands throughout the body.4 Around a acid. Gastrin secretion begins even before food 30% of these tumors are found to be malignant. is consumed, as the mere anticipation of a meal ZES is commonly associated with the MEN1 due can lead to a series of events ending in the to the presence of tumors found in the stomach creation of the gastric acid needed to breakdown and small intestine.4 These gastrointestinal foods. Not only does Gastrin support the tumors caused by MEN 1 give the person a digestion of foods, but it also stimulates growth, higher likelihood of contracting ZES, as the secretion, blood flow, and acts as a defense probability of having pancreatic or duodenal mechanism against bacteria in the tumors is increased.4 gastrointestinal system. When the production of Gastrin becomes overt, major consequences like that of Zollinger Ellison Syndrome (ZES). ZES is an extremely rare disease occurring in people between the ages of 30-60.4 ZES is related to the uncontrolled secretion of gastrin due to the presence of certain pancreatic or duodenal tumors. -

Reciprocal Regulation of Antral Gastrin and Somatostatin Gene Expression by Omeprazole-Induced Achlorhydria
Reciprocal regulation of antral gastrin and somatostatin gene expression by omeprazole-induced achlorhydria. S J Brand, D Stone J Clin Invest. 1988;82(3):1059-1066. https://doi.org/10.1172/JCI113662. Research Article Gastric acid exerts a feedback inhibition on the secretion of gastrin from antral G cells. This study examines whether gastrin gene expression is also regulated by changes in gastric pH. Achlorhydria was induced in rats by the gastric H+/K+ ATPase inhibitor, omeprazole (100 mumol/kg). This resulted in fourfold increases in both serum gastrin (within 2 h) and gastrin mRNA levels (after 24 h). Antral somatostatin D cells probably act as chemoreceptors for gastric acid to mediate a paracrine inhibition on gastrin secretion from adjacent G cells. Omeprazole-induced achlorhydria reduced D-cell activity as shown by a threefold decrease in antral somatostatin mRNA levels that began after 24 h. Exogenous administration of the somatostatin analogue SMS 201-995 (10 micrograms/kg) prevented both the hypergastrinemia and the increase in gastrin mRNA levels caused by omeprazole-induced achlorhydria. Exogenous somatostatin, however, did not influence the decrease in antral somatostatin mRNA levels seen with achlorhydria. These data, therefore, support the hypothesis that antral D cells act as chemoreceptors for changes in gastric pH, and modulates somatostatin secretion and synthesis to mediate a paracrine inhibition on gastrin gene expression in adjacent G cells. Find the latest version: https://jci.me/113662/pdf Reciprocal Regulation of Antral Gastrin and Somatostatin Gene Expression by Omeprazole-induced Achlorhydria Stephen J. Brand and Deborah Stone Departments ofMedicine, Harvard Medical School and Massachusetts General Hospital, Gastrointestinal Unit, Boston, Massachusetts Abstract Substantial evidence supports the hypothesis that gastric acid inhibits gastrin secretion through somatostatin released Gastric acid exerts a feedback inhibition on the secretion of from antral D cells (9, 10). -

(12) Patent Application Publication (10) Pub. No.: US 2003/0198970 A1 Roberts (43) Pub
US 2003O19897OA1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2003/0198970 A1 Roberts (43) Pub. Date: Oct. 23, 2003 (54) GENOSTICS clinical trials on groups or cohorts of patients. This group data is used to derive a Standardised method of treatment (75) Inventor: Gareth Wyn Roberts, Cambs (GB) which is Subsequently applied on an individual basis. There is considerable evidence that a significant factor underlying Correspondence Address: the individual variability in response to disease, therapy and FINNEGAN, HENDERSON, FARABOW, prognosis lies in a person's genetic make-up. There have GARRETT & DUNNER been numerous examples relating that polymorphisms LLP within a given gene can alter the functionality of the protein 1300 ISTREET, NW encoded by that gene thus leading to a variable physiological WASHINGTON, DC 20005 (US) response. In order to bring about the integration of genomics into medical practice and enable design and building of a (73) Assignee: GENOSTIC PHARMA LIMITED technology platform which will enable the everyday practice (21) Appl. No.: 10/206,568 of molecular medicine a way must be invented for the DNA Sequence data to be aligned with the identification of genes (22) Filed: Jul. 29, 2002 central to the induction, development, progression and out come of disease or physiological States of interest. Accord Related U.S. Application Data ing to the invention, the number of genes and their configu rations (mutations and polymorphisms) needed to be (63) Continuation of application No. 09/325,123, filed on identified in order to provide critical clinical information Jun. 3, 1999, now abandoned. concerning individual prognosis is considerably less than the 100,000 thought to comprise the human genome. -

HISTOLOGICAL TYPING of ENDOCRINE TUMOURS INTERNATIONAL HISTOLOGICAL CLASSIFICATION of TUMOURS No
HISTOLOGICAL TYPING OF ENDOCRINE TUMOURS INTERNATIONAL HISTOLOGICAL CLASSIFICATION OF TUMOURS No. 23 HISTOLOGICAL TYPING OF ENDOCRINE TUMOURS E. D. WILLIAMS Head, WHO Collaboratmg Centre for the Histologica/ Classificatton of Endocrine Tumours. · Department of Pathology, The Welsh National School of Medicine, Cardiff. Wales, Umted Kingdom in collaboration with R. E. SIEBENMANN L. H. SOBIN lnstitute of Pathology, Pathologist, Stadtspital Triemli, World Hea/th Orgamzation, Zurich, Switzerland Geneva, Switzer/and and pathologists in 13 countries WORLD HEALTH ORGANIZATION GENEVA 1980 ISBN 92 4 176023 O © World Health Organization 1980 Pubhcations ofthe World Health Orgamzation enjoy copyright protection m accordance with the provisions of Protocol2 of the Universal Copyright Convention. For rights of reproduction or translation of WHO publicatwns, m part or in tofo, application should be made to the Office of Publications, World Health Orgamzatwn, Geneva, Sw1tzerland. The World Health Organi zation welcomes such applications. The designatwns employed and the presentatwn of the material in this publicatwn do not 1mply the expression of any opinion whatsoever on the part of the Duector-General of the World Health Organization concerning the legal status of any country, territory, city or area or of 1ts authorities, or concerning the delimitation of its front1ers or boundanes. The mention of specific compames or of certam manufacturers' products does not imply that they are endorsed or recommended by the W orld Health Organizatwn in preference to others of a similar nature that are not mentioned. Errors and omJsswns excepted, the names of proprietary products are distingmshed by imt1al capitalletters. Authors alone are responsible for v1ews expressed in th1s publication.