Application of Mustard and Its Derivativbs for Control
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Brauhaus Take out Menu Spring Front
Beilagen / sides Spezialitäten / chef’s specialties half 2.5 whole 4 a modern take on german cooking, does not include sides Bratkartoffeln Grüne Spargel fried potatoes / onions green asparagus / shallot Spießbraten – Kartoffelknödel Rotkohl 22 lamb roast / garlic-herb stuffed / spätzle / potato dumpling / sweet & sour red asparagus / mustard-cornich butter cabbage on sauce Pommes Kartoffelsalat Tafelspitzsteak – rench fries 21 potato salad tri-tip steak / foie gras butter / potato puree Spätzle Hausalat grilled asparagus / caperberry sautéed egg dumplings house salad Kartoffelpuffer Forelle – 20 Gurkensalat whole trout / almonds / aspa potato pancake / cucumber-red onion ragus / finger- ling potatoes / c sour cream salad aperberry brown butter Apfelmus Sauerkraut Lachs – 20 applesauce bacon / caraway grilled salmon / potato pancake / asparagus horseradish-creme fraiche / capers zum Teilen / to share 14 Himmel und Äd 17 meant for two to three to be shared at the blutwurst / potato puree / apple gastrique table, served with hearty rye bread Gemüsepfanne – 17 selections may vary, ask your server for a today’s offerings sparagus / goat cheese / potatoes / mush- rooms / spinach / smoked garlic Käse - imported and Fisch - smoked and domestic cheese pickled fish Jägerpfanne – 21 Limburger Lachs roasted pork flat iron / “hunter’s sauce” Aged Gouda Forelle wild mushrooms / fingerling potatoes Camembert Rollmops Bavarian Blue Sour Cream Zigeunerpfanne – 21 roasted pork flat i Almond Honey Capers ron / “gypsy sauce” roasted peppers / fingerling p Optimator Onion Jam Beet Horseradish otatoes Cornichons Pickles - market vegetarian gluten free Schinken - cured fresh pickles and and smoked meats preserves * Consuming raw or undercooked meats, Leberwurst Mixed vegetables poultry, seafood, shellfish, or eggs may increase Salami Red beet egg your risk of foodborne illness, Speck / cured belly Smoked sausage especially if you have a medical condition. -

Use of Deodorized Yellow Mustard Powder to Control Escherichia Coli O157:H7 In
i Use of Deodorized Yellow Mustard Powder to Control Escherichia coli O157:H7 in Dry Cured Westphalian Ham BY Anna Marie Nilson A Thesis submitted to the Faculty of Graduate Studies of The University of Manitoba in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE Department of Food Science University of Manitoba Winnipeg, Manitoba © Copyright by Anna Marie Nilson 2011 Library and Archives Bibliothèque et Canada Archives Canada Published Heritage Direction du Branch Patrimoine de l'édition 395 Wellington Street 395, rue Wellington Ottawa ON K1A 0N4 Ottawa ON K1A 0N4 Canada Canada Your file Votre référence ISBN: 978-0-494-84587-5 Our file Notre référence ISBN: 978-0-494-84587-5 NOTICE: AVIS: The author has granted a non- L'auteur a accordé une licence non exclusive exclusive license allowing Library and permettant à la Bibliothèque et Archives Archives Canada to reproduce, Canada de reproduire, publier, archiver, publish, archive, preserve, conserve, sauvegarder, conserver, transmettre au public communicate to the public by par télécommunication ou par l'Internet, prêter, telecommunication or on the Internet, distribuer et vendre des thèses partout dans le loan, distrbute and sell theses monde, à des fins commerciales ou autres, sur worldwide, for commercial or non- support microforme, papier, électronique et/ou commercial purposes, in microform, autres formats. paper, electronic and/or any other formats. The author retains copyright L'auteur conserve la propriété du droit d'auteur ownership and moral rights in this et des droits moraux qui protege cette thèse. Ni thesis. Neither the thesis nor la thèse ni des extraits substantiels de celle-ci substantial extracts from it may be ne doivent être imprimés ou autrement printed or otherwise reproduced reproduits sans son autorisation. -

Ham and Food Safety
United States Department of Agriculture Food Safety and Inspection Service Food Safety Information PhotoDisc Ham and Food Safety ams: They can be fresh, cook-before-eating, cooked, picnic, and country types. There are so many kinds, Hand their storage times and cooking times vary. This background information serves to carve up the facts and make them easier to understand. Definition Hams may be fresh, cured, or cured-and-smoked. Ham is the cured leg of pork. Fresh ham is an uncured leg of pork. Fresh ham will bear the term “fresh” as part of the product name and is an indication that the product is not cured. “Turkey” ham is a ready-to-eat product made from cured thigh meat of turkey. The term “turkey ham” is always followed by the statement “cured turkey thigh meat.” The usual color for cured ham is deep rose or pink; fresh ham (which is not cured) has the pale pink or beige color of a fresh pork roast; country hams and prosciutto (which are dry cured) range from pink to a mahogany color. Hams are either ready to eat or not. Ready-to-eat hams include prosciutto and cooked hams; they can be eaten right out of the package. Fresh hams and hams that are only treated to destroy trichinae (which may include heating, freezing, or curing in the plant) must be cooked by the consumer before eating. Hams that must be cooked will bear cooking instructions and safe handling instructions. Hams that are not ready-to-eat, but have the appearance of ready-to-eat products, will bear a prominent statement on the principal display panel (label) indicating the product needs cooking, e.g., “cook thoroughly.” In addition, the label must bear cooking directions. -

Ham for the Holidays, December 2007
ISSUE VOLUME DECEMBER 4 16 2017 Hams, Hams, Hams Charts & Terms Ham It Up Ham for the HOLIDAYS Fresh, cook-before-eating, cooked, picnic, from smoldering fires, which gives added country types … knowing how to store and flavor and color to meat and slows the cook different hams can be quite confusing. development of rancidity. Not all smoked meat is smoked from smoldering fires. A Hams may be fresh, cured, or cured-and- popular process is to heat the ham in a smoked. Ham is the cured leg of pork. Fresh smoke house and generate smoke from ham is an uncured leg of pork. Fresh ham will atomized smoke flavor. bear the term "fresh" as part of its name and is an indication that the product is not cured. Quantity to Buy When buying a ham, estimate the size The usual color for cured ham is deep rose or needed according to the number of servings pink; fresh ham (which is not cured) has the the type of ham should yield: pale pink or beige color of a fresh pork roast. ¼ - ⅓ lb. per serving of boneless ham Hams are either ready to eat or not. Ready- ⅓ - ½ lb. of meat per serving of bone- to-eat hams include prosciutto and cooked in ham hams; they can be eaten out of the package. Hams that must be cooked will bear cooking Cooking or Reheating Hams instructions & safe handling methods. Both whole & half, cooked, vacuum-packaged hams packaged in federally inspected plants Hams that are not ready to eat, but have the & canned hams can be eaten cold just as they appearance of ready-to-eat products, will come from their packaging. -

Simplicissimus : the German Adventurer / by Hans Jacob Christoffel Von Grimmelshausen ; Translated by John C
by Hans Jacob Christoffel von Grimmelshausen Simplicissimus,The German Adventurer Translated by JOHN C. OSBORNE With a Foreword by LYNNE TATLOCK Simplicissimus, The German Adventurer by Hans Jacob Christoffel von Grimmelshausen Translated by JOHN C. OSBORNE The GermanWith a Foreword Adventurer by Simplicissimus,LYNNE TATLOCK The German Simplicißimus That is: The Description of the Life of a Peculiar Wanderer Named Melchior Sternfels von Fuchshaim, Including Where and in What Manner He Came into This World, What He Saw, Learned, Experienced and Put Up With Therein; Also Why He Voluntarily Left It. Exceedingly Amusing and in Many Ways Useful to Read. Brought to Light by German Schleifheim of Sulsfort Monpelgart Printed in the Shop of Johann Fillion In the Year 1669. Newfound Press THE UNIVERSITY OF TENNESSEE LIBRARIES, KNOXVILLE Simplicissimus, The German Adventurer © 2008 by Martha Lee Osborne All rights reserved. Newfound Press is a digital imprint of the University of Tennessee Libraries. Its publications are available for non-commercial and educational uses, such as research, teaching and private study. The author encourages the “fair use” of these materials as defined in current U.S. Copyright Law. You may reproduce Newfound Press materials by printing, downloading, or making copies without prior permission as long as the original work is credited. For all other uses, contact: Newfound Press University of Tennessee Libraries 1015 Volunteer Boulevard Knoxville, TN 37996-1000 www.newfoundpress.utk.edu Cover illustration is frontispiece from the 1669 edition, courtesy of the Beinecke Rare Book and Manuscript Library, Yale University. ISBN-13: 978-0-9797292-2-5 ISBN-10: 0-9797292-2-x Grimmelshausen, Hans Jakob Christoph von, 1625-1676. -

Comparison of European & American Systems of Production and Consumption of Dry-Cured Hams
Authors Comparison of European Herbert W. Ockerman, The Ohio State University Lopa Basu, The Ohio State University & American Systems of Production Francesco Leon Crespo, U. of Córdoba, Spain and Consumption of Dry-Cured Hams Francisco J. Céspedes Sánchez, U. of Córdoba, Spain Originally published as a National Pork Board/ Reviewer American Meat Science Association Fact Sheet. Vern Cahill, The Ohio State University Introduction Dry cured hams or jamon have been produced using only pork, sea salt, fresh mountain air and time (Provence, 2002) in Southern Europe for 2000 years. USDA definition of a “ham” is the hind leg of a hog, which may be either fresh, cured, or cured-and smoked (FSIS, 1995). U.S. country (or dry-cured) hams are uncooked, cured, dried, smoked-or-unsmoked and produced from a single piece of meat from the hind leg of a hog (FSIS. 1995). Dry-cured hams and prosciutto are made by rubbing the fresh ham with dry salt and other ingredients which draws out the moisture and then drying for 6 months but most are cured 9-12 months and many over a year which reduces the ham weight by at least 18% (usually 20-35% which is the minimum in Spain) and consequently results in a concentrated ham flavor. Since this drying process also concentrates the salt and reduces the moisture level these hams are safe to store at room temperature. In the U.S. dry-cured hams cannot be injected with a curing solution or placed in a curing solutions, but they may be smoked (FSIS, 1995). -

GERMAN CUISINE Capital
CHEFS https://cisock.wordpress.com/ GERMAN CUISINE Capital: Berlin Currency: Euro Borders: Germany shares borders with Russia, Denmark in the north, Poland and the Czech Republic in the east, Switzerland and Austria in the south, France in the southwest and Belgium, Luxembourg and the Netherlands in the west FEATURES OF GERMAN CUISINE The world war 2 highly influenced the modern German cuisine. German cuisine is famous for its wines, breads, charcuterie products etc. Potatoes forms a large part of the German diet and vegetables like beetroot, cabbage, kohlrabi, turnip, spargel (white asparagus) etc are widely used. Pork, beef and chicken are the commonly used meat, wild boar, duck, goose are also eaten, lamb is rarely used. Braising, stewing, roasting are the main cooking methods. Frying also. Rhine river is the major source of seafood in German cuisine. REGIONS OF GERMANY BAVARIA AND FRANCONIA o It is situated in the south of Germany, these regions has the influence of Swiss cuisine. o Pork is commonly eaten as barbecue or spit roasting style. Eg: spanferkel-baby pig cooked over spit roast. o Other famous dishes fro this region are 'Dresden stollen'(Christmas fruit bread), 'Knodel(Dumplings or soaked bread), 'Haxen' (pork or veal trotter, consumed with sauerkraut), 'Leberknodel' (Liver dumpling in a clear broth). LOWER SAXONY AND SCHLESWIG-HOLSTEIN o These regions lies in the north-west Germany facing the North sea. Rich in seafood like herring, eel etc. o Has an influence of Scandinavian countries. o Sweet and sour eel soup flavoured with bacon called 'Aalsuppe' is a famous dish from this region. -

T.C. Akdeniz Üniversitesi Kurutulmuş Et Ürünü
T.C. AKDENİZ ÜNİVERSİTESİ KURUTULMUŞ ET ÜRÜNÜ ÜRETMEK İÇİN SOĞUK KURUTUCU TASARIMI VE ELDE EDİLEN KURU ET ÜRÜNÜNÜN KURUMA-KALİTE KARAKTERİSTİKLERİNİN BELİRLENMESİ Elif AYKIN DİNÇER FEN BİLİMLERİ ENSTİTÜSÜ GIDA MÜHENDİSLİĞİ ANABİLİM DALI DOKTORA TEZİ EKİM 2018 ANTALYA T.C. AKDENİZ ÜNİVERSİTESİ KURUTULMUŞ ET ÜRÜNÜ ÜRETMEK İÇİN SOĞUK KURUTUCU TASARIMI VE ELDE EDİLEN KURU ET ÜRÜNÜNÜN KURUMA-KALİTE KARAKTERİSTİKLERİNİN BELİRLENMESİ Elif AYKIN DİNÇER FEN BİLİMLERİ ENSTİTÜSÜ GIDA MÜHENDİSLİĞİ ANABİLİM DALI DOKTORA TEZİ EKİM 2018 ANTALYA T.C. AKDENİZ ÜNİVERSİTESİ FEN BİLİMLERİ ENSTİTÜSÜ KURUTULMUŞ ET ÜRÜNÜ ÜRETMEK İÇİN SOĞUK KURUTUCU TASARIMI VE ELDE EDİLEN KURU ET ÜRÜNÜNÜN KURUMA-KALİTE KARAKTERİSTİKLERİNİN BELİRLENMESİ Elif AYKIN DİNÇER GIDA MÜHENDİSLİĞİ ANABİLİM DALI DOKTORA TEZİ Bu tez Akdeniz Üniversitesi Bilimsel Araştırma Projeleri Koordinasyon Birimi tarafından FBA-2016-1187 nolu proje ile desteklenmiştir. EKİM 2018 T.C. AKDENİZ ÜNİVERSİTESİ FEN BİLİMLERİ ENSTİTÜSÜ KURUTULMUŞ ET ÜRÜNÜ ÜRETMEK İÇİN SOĞUK KURUTUCU TASARIMI VE ELDE EDİLEN KURU ET ÜRÜNÜNÜN KURUMA-KALİTE KARAKTERİSTİKLERİNİN BELİRLENMESİ Elif AYKIN DİNÇER GIDA MÜHENDİSLİĞİ ANABİLİM DALI DOKTORA TEZİ Bu tez …./…../201….tarihinde aşağıdaki jüri tarafından Oybirliği/Oyçokluğu ile kabul edilmiştir. Prof. Dr. Mustafa ERBAŞ (Danışman) Prof. Dr. Mükerrem KAYA Prof. Dr. Ayhan TOPUZ Prof. Dr. Nesimi AKTAŞ Doç. Dr. Mustafa Kemal USLU ÖZET KURUTULMUŞ ET ÜRÜNÜ ÜRETMEK İÇİN SOĞUK KURUTUCU TASARIMI VE ELDE EDİLEN KURU ET ÜRÜNÜNÜN KURUMA-KALİTE KARAKTERİSTİKLERİNİN BELİRLENMESİ Elif AYKIN DİNÇER Doktora Tezi, Gıda Mühendisliği Anabilim Dalı Danışman: Prof. Dr. Mustafa ERBAŞ Ekim 2018; 121 sayfa Bu çalışmada; minimal işlem görmüş ve gıda güvenliği açısından risk oluşturmayan kurutulmuş bir et ürünü üretmek için düşük sıcaklıkta kurutma yapan bir kurutucu tasarlanması ve bu kurutucudan elde edilen ürünün kalite karakteristiklerinin belirlenmesi amaçlanmıştır. -

Beef Lamb and Rabbit Fresh Cut Iqf Grains and Legumes Pasta Ferment Fungi and Tofu Microgreens Sweeteners
CORPORATIONB HEADWATER FOOD HUB 2018/2019 PRODUCT CATALOG Delivering fresh, sustainable, local food to chefs, institutions, retail and more! 585.313.7670 HEADWATERFOODHUB.COM TABLE OF CONTENTS ABOUT HEADWATER FOOD HUB REGIONAL COLLABORATION OUR SUSTAINABLE FUTURE GOOD FOOD CRITERIA TOOLS FOR CHANGE YEAR ROUND PRODUCTS EGGS CHEESES YOGURT POULTRY PORK SAUSAGES AND CHARCUTERIE BEEF LAMB AND RABBIT FRESH CUT IQF GRAINS AND LEGUMES PASTA FERMENT FUNGI AND TOFU MICROGREENS SWEETENERS SEASONAL PRODUCE SPRING SUMMER FALL WINTER HISTORY THE GOOD FOOD COLLECTIVE OUR TEAM [email protected] 585.313.7670 headwaterfoodhub.com HEADWATER FOOD HUB A NETWORK OF SUSTAINABLE FARMERS & CUSTOMERS, ONE TRUSTED PARTNER. HEADWATER FOOD HUB Headwater Food Hub works collaboratively with a network of regional farmers and food producers to coordinate a Good Food System that delivers top-quality, sustainable foods year-round. For our Customer Partners, we provide a one-stop-shop for this area’s best produce, meat, dairy, and value-added goods. For our Farmer and Food Producer Partners, we provide a consistent, large, and fair market for their products. Headwater Food Hub is connecting the dots of Farm to Table and brings transparency and integrity to the food supply chain. We are committed to a Good Food System that positively impacts our Community’s Health and provides our customers with the best Good Food options possible. [email protected] 585.313.7670 headwaterfoodhub.com REGIONAL COLLABORATION Headwater Food Hub is developing strategic relationships with like-minded Food Hubs in other areas of the region. We seek to help build a Network of Food Hubs that can deliver the volume, continuity, and diversity our customers desire. -

Acetic Acid, 302 Acids, in Sausages, 302 Additives
Index Acetic acid, 302 curing methods, 72, 77 Acids, in sausages, 302 jowl, 334, 349 Additives: nitrite/nitrate levels in, 57 curing, 53-64, 155 nitrosamines in, 58, 59 sausage, 298-302, 308-309 nutritive value of, 32-33, 40, 41, 46 sectioned and formed meat product, production volume of, 13, 16 155-156 smoking of, 81 Aging, 108 spices in, 321 Air-conditioned smokehouses, 90-91 Wiltshire, 348-349 Alcohols, in smoke, 83 Bacteria: Alginates, 308, 359-360 in fermentation, 230-231, 250, 256, Allspice, 313, 327 309 Aluminum cans, 378-379 pathogenic, 48, 56-57, 81, 83, \09, 156, American Spice Trade Association 325, 328-331, 372, 384 (ASTA), 324, 331 Baked ham, 334, 340 Animal casings, 291-294 Barbecue sauce, spices in, 321 Animal proteins, 364-365 Beef: Animal shortenings, 16 bacon, 136, 349 Antioxidants, 308-309, 316, 328-331, consumer expenditures on, 8 416 corned, 18,41,46,72-73, 136, 161, 163, Appert, Nicholas, 1 333,334,350-3:,)1,373,374 Arachidonic acid, 28 cured, 13, 46 Artery pumping, 74-75 dark cutting, 140-141 Ascorbates, 59, 61-62, 300 dried, 13, 18,46, 136,334,351-352,373, Ascorbic acid: 374, 402-403 as a curing ingredient, 58-59, 61-62 fresh, 8, 14,26,44, 140-141 in meat products, 33, 34, 42, 44-47 frozen boneless rolls for grilling or broil- Ash content, 25-27, 29-32, 40, 41 ing, 122-123 hash, 373, 374 myoglobin content, 65 Baby foods: nutritive value of, 26, 28, 34, 40, 41, nitrite/nitrate levels in, 57 44 nutritive value of, 30, 32-34, 39, 40 production of, 3, 4, 6, 7, 13 Bacon, 334, 347-350 restructured products, 425-428, -

Charcuterie Professional Certification (CPC) Examination‐ Certified Salumiere Content Outline
Charcuterie Professional Certification (CPC) Examination‐ Certified Salumiere Content Outline The content outline is a delineation of the major domains of practice, tasks performed, and knowledge applied, by a charcuterie professional in the practice of their profession. A charcuterie professional is an individual with knowledge of the production, marketing, handling, and characteristics of charcuterie. The charcuterie professional understands business, safety and sanitation practices associated with the sale of charcuterie. Educates customers on individual product attributes (e.g., origin, production methods) to facilitate sales and to meet the needs of the customers (e.g., retailer, restaurant, consumer). The Charcuterie Professional Certification (CPC) Examination consists of 100 multiple‐choice test questions distributed among the major domains of practice as presented below: Percentage of Test Major Domains of Practice Questions I. Preparation of Charcuterie Products for Merchandising 8% II. Marketing/Sales, including Customer Service, Merchandising 42% III. Product Handling 28% IV. Business Practices and Finance 7% V. Safety and Sanitation Regulations 15% Total 100% DOMAIN I. Preparation of Charcuterie Products for Merchandising Task 1.1. Wash and brush product to properly remove mold and maintain appearance and flavor in order for the product to be appealing to customers (e.g., restaurants, food service/catering, and retail). The safe and effective performance of this task requires knowledge of: K‐1. The difference between good and bad mold K‐2. Sound sanitation practices Task 1.2. Remove the casing to make the product appealing to the customer when appropriate. The safe and effective performance of this task requires knowledge of: K‐3. Differences in casings (e.g., edible vs. -
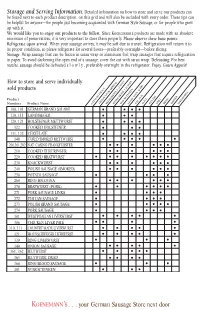
Storage and Serving Information.Detailed Information on How to Store and Serve Our Products
Grid.qxd 12/13/01 10:51 AM Page 2 Storage and Serving Information. Detailed information on how to store and serve our products can be found next to each product description, on this grid and will also be included with every order. These tips can be helpful for anyone—for people just becoming acquainted with German Style Sausage, or for people who grew up with it. We would like you to enjoy our products to the fullest. Since Koenemann’s products are made with an absolute minimum of preservatives, it is very important to store them properly. Please observe these basic points: Refrigerate upon arrival. When your sausage arrives, it may be soft due to transit. Refrigeration will return it to its proper condition, so please refrigerate for several hours—preferably overnight—before slicing. Storage: Wrap sausage that can be frozen in saran wrap or aluminum foil; wrap sausages that require refrigeration in paper. To avoid darkening the open end of a sausage, cover the cut with saran wrap. Defrosting: For best results, sausage should be defrosted s l o w l y , preferably overnight in the refrigerator. Enjoy. Guten Appetit! How to store and serve individually sold products Product Numbers Product Name CATALOG PAGEFREEZE # UPONREFRIGERATE ARRIVALREFRIGERATE 1-21 DAYSMAY BE1-14 FROZEN DAYSSTORE COOLEAT & AS DRY ISSLICE PAN FRY GRILL BROIL SIMMER SPREADABLE 100, 101 GERMAN BRAND SALAMI •••• 110, 111 LANDJAEGER ••• 120, 121 HOLSTEINER METTWURST •••• 122 COOKED HOLSTEINER ••• 131, 133 CERVELATE •••• 140 CURED SMOKED METTWURST ••••• 200,201,202 NAT.