KDC470 Barcode/RFID/Mpos Smartsled
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ITG Barcode Generator
ITG Barcode Generator Copyright © 2007-2018, IT Genetics. All Rights Reserved. 3 Contents Introduction 5 1 Key Fe.a..t.u..r..e..s......................................................................................................................... 5 2 System.. .R..e..q..u..i.r.e..m...e..n..t.s............................................................................................................ 6 3 Installi.n..g................................................................................................................................ 6 4 What c.a..n.. .y..o..u.. .d..o.................................................................................................................... 6 How to Generate Barcode Labels 7 1 Genera..t.e.. .L..i.s..t........................................................................................................................ 7 2 Forma.t.t.i.n..g.. .B..a..r.c..o..d..e............................................................................................................... 9 Printing Barcodes 9 1 Printin.g.................................................................................................................................. 9 2 Chang..i.n..g.. .P...r.i.n..t.e..r. .S..e..t.t.i.n..g..s.................................................................................................... 11 Selecting Label Type 11 1 Label. .T..y..p..e..s. .S...u..p..p..o..r.t.e..d........................................................................................................ 14 Symbologies -

Barcode Symbology Reference Guide a Guide to Assist with Selecting the Barcode Symbology
omni-id.com Barcode Symbology Reference Guide A guide to assist with selecting the barcode symbology This document Provides background information pertaining to the major barcode symbologies to allow the reader to understand the features of the codes. Barcode Symbology Reference Guide omni-id.com Contents Introduction 3 Code 128 4 Code 39 4 Code 93 5 Codabar (USD-4, NW-7 and 2OF7 Code) 5 Interleaved 2 of 5 (code 25, 12OF5, ITF, 125) 5 Datamatrix 5 Aztec Codd 6 QR Code 6 PDF-417 Standard and Micro 7 2 Barcode Symbology Reference Guide omni-id.com Introduction This reference guide is intended to provide some guidance to assist with selecting the barcode symbology to be applied to the Omni-ID products during Service Bureau tag commissioning. This document Provides background information pertaining to the major barcode symbologies to allow the reader to understand the features of the codes. This guide provides information on the following barcode symbologies; • Code 128 (1-D) • Code 39 (1-D) • Code 93 (1-D) • Codabar (1-D) • Interleave 2of5 (1-D) • Datamatrix (2-D) • Aztec code (2-D) • PDF417-std and micro (2-D) • QR Code (2-D) 3 Barcode Symbology Reference Guide omni-id.com Code 128 Code 128 is one of the most popular barcode selections. Code 128 provides excellent density for all-numeric data and good density for alphanumeric data. It is often selected over Code 39 in new applications because of its density and because it offers a much larger selection of characters. The Code 128 standard is maintained by AIM (Automatic Identification Manufacturers). -

RP2/RP4* Mobile Printers
RP2/RP4* Mobile Printers User Guide * For China, models RP2B-C, RP2D-C, RP4B, RP4D-C *For Thailand models RP2B, RP2D-T, RP4B, RP4D-T *For India models RP2B, RP2D, RP4B-I, RP4D-I Disclaimer Honeywell International Inc. (“HII”) reserves the right to make changes in specifications and other information contained in this document without prior notice, and the reader should in all cases consult HII to determine whether any such changes have been made. The information in this publication does not represent a commitment on the part of HII. HII shall not be liable for technical or editorial errors or omissions contained herein; nor for incidental or consequential damages resulting from the furnishing, performance, or use of this material. HII disclaims all responsibility for the selection and use of software and/or hardware to achieve intended results. This document contains proprietary information that is protected by copyright. All rights are reserved. No part of this document may be photocopied, reproduced, or translated into another language without the prior written consent of HII. Copyright 2017-2020 Honeywell International Inc. All rights reserved. Web Address: www.honeywellaidc.com. Trademarks Microsoft Windows 7, Windows 8, Windows Mobile, and Windows CE are trademarks or registered trademarks of Microsoft Corporation. Wavelink Avalanche is a registered trademark of Wavelink Corporation. The Bluetooth word mark and logos are owned by Bluetooth SIG, Inc. Android is a trademark of Google Inc. Other product names or marks mentioned in this document may be trademarks or registered trademarks of other companies and are the property of their respective owners. -

Useful Facts About Barcoding
Useful Facts about Barcoding When Did Barcodes Begin? (Part 1) A barcode is an optical machine-readable representation of data relating to the object to which it is attached. Originally barcodes represented data by varying the widths and spacing’s of parallel lines and may be referred to as linear or one-dimensional (1D). Later they evolved into rectangles, dots, hexagons and other geometric patterns in two dimensions (2D). Although 2D systems use a variety of symbols, they are generally referred to as barcodes as well. Barcodes originally were scanned by special optical scanners called barcode readers; later, scanners and interpretive software became available on devices including desktop printers and smartphones. Barcodes are on the leading edge of extraordinary things. They have given humans the ability to enter and extract large amounts of data in relatively small images of code. With some of the latest additions like Quick Response (QR) codes and Radio-frequency identification (RFID), it’s exciting to see how these complex image codes are being used for business and even personal use. The original idea of the barcode was first introduced in 1948 by Bernard Silver and Norman Joseph Woodland after Silver overheard the President of a local food chain talking about their need for a system to automatically read product information during checkout. Silver and Woodland took their inspiration from recognizing this rising need and began development on this product so familiar to the world now. After several attempts to create something usable, Silver and Woodland finally came up with their ”Classifying Apparatus and Method” which was patented on October 07, 1952. -

Programming Guide 1400 10Th Street Plano, TX 75074 0308 US CCD LR Programming Guide Wasp Barcode Technologies
Barcode Scanning Made Easy Wasp Barcode Technologies Programming Guide 1400 10th Street Plano, TX 75074 www.waspbarcode.com 0308 US CCD LR Programming Guide Wasp Barcode Technologies Please Read Note: The Wasp® WLR8900 Series Scanners are ready to scan the most popular barcodes out of the box. This manual should only be used to make changes in the configuration of the scanner for specific applications. These scanners do not require software or drivers to operate. The scanner enters data as keyboard data. Please review this manual before scanning any of the programming barcodes in this manual. Tech Tip If you are unsure of the scanner configuration or have scanned the incorrect codes, please scan the default barcode on page 7. This will reset the scanner to its factory settings. Check Version Productivity Solutions for Small Business that Increases Productivity & Profitability • Barcode, data colection solutions • Small business focus • Profitable growth since 1986 • Over 200,000 customers • Business unit of Datalogic SPA © Copyright Wasp Barcode Technologies 2008 No part of this publication may be reproduced or transmitted in any form or by any Wasp® Barcode Technologies means without the written permission of Wasp Barcode Technologies. The information 1400 10th Street contained in this document is subject to change without notice. Plano, TX 75074 Wasp and the Wasp logo are registered trademarks of Wasp Barcode Technologies. All other Phone: 214-547-4100 • Fax: 214-547-4101 trademarks or registered trademarks are the property of their respective owners. www.waspbarcode.com WLR8900_8905Manual0308_sm.A0 6/25/08 3:38 PM Page 1 Table of Contents Chapter 1. -

IGP® / VGL Emulation Code V™ Graphics Language Programmer's Reference Manual Line Matrix Series Printers
IGP® / VGL Emulation Code V™ Graphics Language Programmer’s Reference Manual Line Matrix Series Printers Trademark Acknowledgements IBM and IBM PC are registered trademarks of the International Business Machines Corp. HP and PCL are registered trademarks of Hewlett-Packard Company. IGP, LinePrinter Plus, PSA, and Printronix are registered trademarks of Printronix, LLC. QMS is a registered trademark and Code V is a trademark of Quality Micro Systems, Inc. CSA is a registered certification mark of the Canadian Standards Association. TUV is a registered certification mark of TUV Rheinland of North America, Inc. UL is a registered certification mark of Underwriters Laboratories, Inc. This product uses Intellifont Scalable typefaces and Intellifont technology. Intellifont is a registered trademark of Agfa Division, Miles Incorporated (Agfa). CG Triumvirate are trademarks of Agfa Division, Miles Incorporated (Agfa). CG Times, based on Times New Roman under license from The Monotype Corporation Plc is a product of Agfa. Printronix, LLC. makes no representations or warranties of any kind regarding this material, including, but not limited to, implied warranties of merchantability and fitness for a particular purpose. Printronix, LLC. shall not be held responsible for errors contained herein or any omissions from this material or for any damages, whether direct, indirect, incidental or consequential, in connection with the furnishing, distribution, performance or use of this material. The information in this manual is subject to change without notice. This document contains proprietary information protected by copyright. No part of this document may be reproduced, copied, translated or incorporated in any other material in any form or by any means, whether manual, graphic, electronic, mechanical or otherwise, without the prior written consent of Printronix, LLC. -
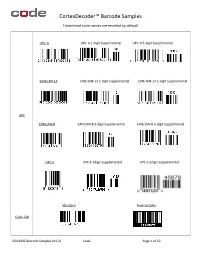
Cortexdecoder™ Barcode Samples
CortexDecoder™ Barcode Samples Underlined code names are enabled by default UPC-A UPC-A 2-digit Supplemental UPC-A 5-digit Supplemental EAN/JAN-13 EAN/JAN-13 2-digit Supplemental EAN/JAN-13 5-digit Supplemental UPC EAN/JAN-8 EAN/JAN-8 2-digit Supplemental EAN/JAN-8 5-digit Supplemental UPC-E UPC-E 2digit Supplemental UPC-E 5digit Supplemental Standard Inverse Color Code 128 D014402 Barcode Samples (V4.2) Code Page 1 of 12 CortexDecoder™ Barcode Samples Underlined code names are enabled by default Standard Inverse Color Code 39 Checksum Full ASCII Standard Checksum Interleaved 2 of 5 Standard Mod16 Checksum 7DR Checksum Codabar (NW-7) By default, Start/Stop chars are displayed in output. Code 93 D014402 Barcode Samples (V4.2) Code Page 2 of 12 CortexDecoder™ Barcode Samples Underlined code names are enabled by default GS1 DataBar Omni/Truncated GS1 DataBar Stacked/Stacked Omni GS1 DataBar Limited GS1- GS1 DataBar Expanded GS1 DataBar Expanded Stacked DataBar The only difference between Omni(directional) and Truncated is that the bar height is taller for Omni and shorter for Truncated. GS1 DataBar Stacked implies it is truncated. EAN/JAN-8 with CC-A EAN/JAN-13 with CC-A 1234567021A12345678 3312345678903991234-abcd DataBar Limited with CC-B DataBar Limited with CC-A GS1- Composite 01131123456789061701061510A123456 010351234567890721abcdefghijklmnopqrstuv GS1-128 with CC-C GS1 DataBar and GS1-128 as part of the composite contains a link character indicating the existence of the composite code. EAN/JAN does not contain such a link. Therefore it is normal that the above EAN/JAN CCA samples may output the 1D only when the composite is not decodable. -

WDI4600 2D Barcode SCANNER
White Technologies Business Card Logo Wasp Logo Reversed Bee Only Regular Logo PMS116 CVC Regular Logo 4C Logo with Black Wasp WDI4600 2D BARCODE SCANNER Logo Outline Wasp Box Logo Productivity Solutions for Small Businesses The Wasp WDI4600 barcode scanner rapidly reads 1D and 2D barcodes, including QR codes. The WDI4600 streamlines data entry and allows you to instantly add barcode data to spreadsheets, word documents, and databases. Use the WDI4600 to improve efficiency and eliminate data-entry errors in healthcare, retail, office, shipping and receiving, travel, hospitality, manufacturing, and more. Installing and using the WDI4600 is fast and easy. Connect the WDI4600 directly to your PC’s USB port, and you’re ready to begin scanning 1D and 2D barcodes. A built-in, 4-Dot aiming guide sets a precise reading zone and reduces accidental reads. The laser’s center cross offers a targeted scanning when multiple barcodes are in view and allows for the reading of barcodes on mobile devices. The WDI4600 works with or without the use of a stand and automatically switches to the desired scanning mode. zx WASP WDI4600 FEATURES zx BENEFITS Read 1D barcodes up to 15” away and 2D barcodes up to 10” away Rapidly add 1D and 2D barcode data to documents, spreadsheets, and Withstands multiple 6’ drops to concrete databases Compatible with optional hands-free stand for autosense scanning Improve productivity and prevent data-entry errors Ergonomic design minimizes user fatigue Omnidirectional reading Reads all common 1D barcodes and 2D barcodes; including -

CK65 Series Mobile Computer User Guide, Powered By
CK65 Series Mobile Computer powered by Android™ User Guide Disclaimer Honeywell International Inc. (“HII”) reserves the right to make changes in specifications and other information contained in this document without prior notice, and the reader should in all cases consult HII to determine whether any such changes have been made. The information in this publication does not represent a commitment on the part of HII. HII shall not be liable for technical or editorial errors or omissions contained herein; nor for incidental or consequential damages resulting from the furnishing, performance, or use of this material. HII disclaims all responsibility for the selec- tion and use of software and/or hardware to achieve intended results. This document contains proprietary information that is protected by copyright. All rights are reserved. No part of this doc- ument may be photocopied, reproduced, or translated into another language without the prior written consent of HII. Copyright 2019 Honeywell International Inc. All rights reserved. Web Address: www.honeywellaidc.com Trademarks Google and Android are trademarks of Google LLC. Bluetooth trademarks are owned by Bluetooth SIG, Inc., U.S.A. and licensed to Honeywell. microSD is a registered trademark of SD-3C, LLC. Qualcomm and Snapdragon are registered trademarks or trademarks of Qualcomm Incorporated in the United States and/or other countries. Other product names or marks mentioned in this document may be trademarks or registered trademarks of other compa- nies and are the property of their respective owners. Patents For patent information, refer to www.hsmpats.com. TABLE OF CONTENTS Customer Support ....................................................................................................................... vii Technical Assistance ............................................................................................................vii Product Service and Repair .............................................................................................. -

BCBP Implementation Guide Is Intended to Be Used As Guidance Material When Airlines Would Like to Implement Bar Coded Boarding Pass (BCBP)
BAR CODED BOARDING PASS (BCBP) IMPLEMENTATION GUIDE Effective Date: 1st June, 2018 Seven Edition Page | 1 DISCLAIMER. The information contained in this publication is subject to constant review in the light of changing government requirements and regulations. No reader should act on the basis of any such information without referring to applicable laws and regulations and/or without taking appropriate professional advice. Although every effort has been made to ensure accuracy, the International Air Transport Association shall not be held responsible for loss or damage caused by errors, omissions, misprints or misinterpretation of the contents hereof. Furthermore, the International Air Transport Association expressly disclaims all and any liability to any person, whether a purchaser of this publication or not, in respect of anything done or omitted, and the consequences of anything done or omitted, by any such person in reliance on the contents of this publication. No part of the Common Use Passenger Processing Systems Implementation Guide may be reproduced, recast, reformatted or transmitted in any form by any means, electronic or mechanical, including photocopying, recording or any information storage and retrieval system, without the prior written permission from: International Air Transport Association 800 Place Victoria, P.O. Box 113 Montreal, Quebec, Canada H4Z 1M1 © IATA 2017 Page | 2 TABLE OF CONTENTS 1. INTRODUCTION ..................................................................................... 6 1.1. Background .................................................................................................................. -

User Guide 7010 Thermal Printer
User Guide 7010 Thermal Printer 7010 7010R 7010-300 CONTENTS Before Operation INTRODUCTION - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 3 COMPLIANCE STATEMENT FOR EUROPEAN USERS - - - - - - - - - - - - - - - - - - 4 FCC COMPLIANCE STATEMENT FOR AMERICAN USERS - - - - - - - - - - - - - - 4 EMI COMPLIANCE STATEMENT FOR CANADIAN USERS- - - - - - - - - - - - - - - 5 ETAT DE CONFORMITE EMI A L’USAGE DES UTILISATEURS CANADIENS - 5 IMPORTANT SAFETY INSTRUCTIONS - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 6 NOTICE - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 7 SAFETY INSTRUCTIONS - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 8 Chapter 1 Setup Confirmation of Carton Contents - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 10 Part Names and Functions - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 11 Connection to Power - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 18 Driver Installation - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 18 Connection to a Computer - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 19 Chapter 2 Printer Operation Power ON/OFF - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 20 Normal Operating Mode - - - - - - - - - - - - - - - - - - - - - - - - - -

Standard for Serialisation and Data Matrix Codes on Medicines Guidance for TGO 106
Standard for serialisation and data matrix codes on medicines Guidance for TGO 106 Version 1.0, March 2021 Therapeutic Goods Administration Copyright © Commonwealth of Australia 2021 This work is copyright. You may reproduce the whole or part of this work in unaltered form for your own personal use or, if you are part of an organisation, for internal use within your organisation, but only if you or your organisation do not use the reproduction for any commercial purpose and retain this copyright notice and all disclaimer notices as part of that reproduction. Apart from rights to use as permitted by the Copyright Act 1968 or allowed by this copyright notice, all other rights are reserved and you are not allowed to reproduce the whole or any part of this work in any way (electronic or otherwise) without first being given specific written permission from the Commonwealth to do so. Requests and inquiries concerning reproduction and rights are to be sent to the TGA Copyright Officer, Therapeutic Goods Administration, PO Box 100, Woden ACT 2606 or emailed to <[email protected]>. Standard for serialisation and data matrix codes on medicines Page 2 of 18 Guidance for TGO 106 V1.0 March 2021 Therapeutic Goods Administration Contents About this guidance ____________________________ 4 About TGO 106 ________________________________ 4 Commencement date __________________________________________________________ 4 Medicines that are subject to TGO 106 requirements _ 4 Medicines that are exempt from TGO 106 ___________ 5 Export only medicines