Standard for Serialisation and Data Matrix Codes on Medicines Guidance for TGO 106
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Barcode Symbology Reference Guide a Guide to Assist with Selecting the Barcode Symbology
omni-id.com Barcode Symbology Reference Guide A guide to assist with selecting the barcode symbology This document Provides background information pertaining to the major barcode symbologies to allow the reader to understand the features of the codes. Barcode Symbology Reference Guide omni-id.com Contents Introduction 3 Code 128 4 Code 39 4 Code 93 5 Codabar (USD-4, NW-7 and 2OF7 Code) 5 Interleaved 2 of 5 (code 25, 12OF5, ITF, 125) 5 Datamatrix 5 Aztec Codd 6 QR Code 6 PDF-417 Standard and Micro 7 2 Barcode Symbology Reference Guide omni-id.com Introduction This reference guide is intended to provide some guidance to assist with selecting the barcode symbology to be applied to the Omni-ID products during Service Bureau tag commissioning. This document Provides background information pertaining to the major barcode symbologies to allow the reader to understand the features of the codes. This guide provides information on the following barcode symbologies; • Code 128 (1-D) • Code 39 (1-D) • Code 93 (1-D) • Codabar (1-D) • Interleave 2of5 (1-D) • Datamatrix (2-D) • Aztec code (2-D) • PDF417-std and micro (2-D) • QR Code (2-D) 3 Barcode Symbology Reference Guide omni-id.com Code 128 Code 128 is one of the most popular barcode selections. Code 128 provides excellent density for all-numeric data and good density for alphanumeric data. It is often selected over Code 39 in new applications because of its density and because it offers a much larger selection of characters. The Code 128 standard is maintained by AIM (Automatic Identification Manufacturers). -

Tracking Codes and How They Work
Tracking Codes and How They Work > Industrial Traceability 1 Introduction In the past few years, traceability has become a major issue for the industrial sector, since allowing for better tracking and management of products can lead to important cost/savings. Most of the time, this notion of traceability takes the form of barcodes on products. Originally, the well-known, one-dimensional (1D) barcodes were the first barcodes to be created and they have been used ever since due to their simplicity. But due to the limited quantity of information which can be stored in these initial barcodes, a database is needed to interpret the decoded information and to link it to the information of the product. Without the database, the number that is decoded does not mean anything. However, sometimes, a higher density storage of information than the one allowed by 1D codes is needed. So, two-dimensional barcodes were created to store a maximum of information without requiring an accompanying database. State of the Art Code By having the capacity to store information in two-dimensions (2D); these barcodes can store such a density of information that a product and its information can be decoded without using an external database. The code itself can contain information like: the brand, the name of the product, the year of fabrication and so forth. For a given industry, the ability to access this critical information at every step of the production process without the use of an accompanying database greatly facilitates the handling of the product. However, for these codes to be readable by all the subcontractors along the production line, standards for two-dimensional and one-dimensional barcodes needed to be created. -

EIGP 114.2018 (Revision 20 June 2018 Vern Lorenson – ECIA 2D Barcode SME)
ECIA Publication Labeling Specification for Product and Shipment Identification in the Electronics Industry - 2D Barcode (Including Human Readable and 1D Barcode) EIGP 114.2018 (Revision 20 June 2018 Vern Lorenson – ECIA 2D Barcode SME) June 2018 Electronic Components Industry Association Industry Specifications Rev20.06.2018 EIGP 114.2018 Page 1 of 44 NOTICE ECIA Industry Guidelines and Publications contain material that has been prepared, progressively reviewed, and approved through various ECIA-sponsored industry task forces, comprised of ECIA member distributors, manufacturers, and manufacturers’ representatives. After adoption, efforts are taken to ensure widespread dissemination of the guidelines. ECIA reviews and updates the guidelines as needed. ECIA Industry Guidelines and Publications are designed to serve the public interest, including electronic component distributors, manufacturers and manufacturers’ representatives through the promotion of uniform and consistent practices between manufacturers, distributors, and manufacturers’ representatives resulting in improved efficiency, profitability, product quality, safety, and environmentally responsible practices. Existence of such guidelines shall not in any respect preclude any member or non-member of ECIA from adopting any other practice not in conformance to such guidelines, nor shall the existence of such guidelines preclude their voluntary use by those other than ECIA members, whether the guideline is to be used either domestically or internationally. ECIA does not assume any liability or obligation whatever to parties adopting ECIA Industry Guidelines and Publications. Each company must independently assess whether adherence to some or all of the guidelines is in its own best interest. Inquiries, comments, and suggestions relative to the content of this ECIA Industry Guideline should be addressed to ECIA headquarters. -

KDC470 Barcode/RFID/Mpos Smartsled
KDC470 Barcode/RFID/mPOS SmartSled Our Most Modular Product Yet Whether you need to read barcodes or RFID tags this is the KDC for you. The KDC470 is rugged device with an IP65 rating and is built to last. No matter what type of data you need to collect or how you need to collect it, there is a KDC470 module to get the job done quickly and accurately. Charge your device with our unique charging cases and never miss a minute of productivity. Attach to ANY Smartphone or Tablet The KDC470 attaches to any smart device via a custom case to create a sled scanning solution. This unique modular design allows you to upgrade your smart device without worrying about replacing the entire scanning solution. Your investment in a KDC470 is protected regardless of upgrades in smartphone and tablet technology. Barcode Reading At it’s base, the KDC470 is a superior barcode scanner. The Additional Companions KDC470 comes in three different models, 1D Laser, 1D CCD, • Extended Battery Pack - For long shifts or projects. Never and 2D Imager so you can read a variety of barcodes in any worry about the battery of your KDC. industry. The KDC connects via Bluetooth Classic technology • Pistol Grip - Pull the trigger on easy scanning. for easy pairing and data transfers. RFID Companions The RFID companions attach to your KDC470 alllowing for various transactions to be performed via radio frequency identification. The contactless interface can be utilized for asset management and tracking whether those assets are people, animals, or inanimate objects. Options include High Frequency (HF), 0.5W Ultra High Frequency (UHF), or 1.0W UHF. -

Putting QR Codes to Work
Caslon, a PODi Affiliate (Quick Response) Putting QR Codes to Work Steven England Mobile Consultant / Director of Business Development New Media Marketing Who is PODi? Who is Caslon? PODi Mission: Help members build & grow successful businesses using digital print • Printing and Marketing service providers, direct mailers & agencies • Enterprise companies • Consultants and educational organizations • Hardware and software solution providers • Regional industry vendors Caslon, a PODi Affiliate • Caslon creates cutting edge information and resources for Service Providers and Marketers • Manages and builds our community under license from PODi • Develops Case Studies, S3 sales tools, Find a Service Provider, and hosts DEX User Forums and our annual AppForum. Caslon, a PODi Affiliate What can PODi do for YOUR digital business? – Get more leads & promote your company • Connect to customers with Find a Service Provider • Self-Promo-in-a-Box lead generation campaign • Boost your reputation with a Best Practices Award, case study or PODi logo – Increase high-margin business & sell successfully • Energize sales with Digital Print Case Studies • Close more sales with proven S3 Council sales tools & NEW training modules • Learn at free monthly webinars. Plan new strategies with industry reports • NEW Caslon’s DEX S3 Forum – Boost your POD efficiency • NEW Production Central: one-stop resource center for technology support • NEW Caslon’s DEX Tech Forums: HP/Indigo, Kodak, Xerox • NEW Technology Webinars • PPML & CheckPPML_Pro – Save money on expert -
Cortexdecoder™ Barcode Samples
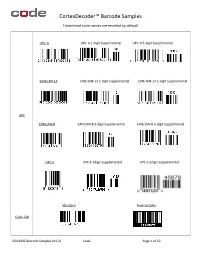
CortexDecoder™ Barcode Samples Underlined code names are enabled by default UPC-A UPC-A 2-digit Supplemental UPC-A 5-digit Supplemental EAN/JAN-13 EAN/JAN-13 2-digit Supplemental EAN/JAN-13 5-digit Supplemental UPC EAN/JAN-8 EAN/JAN-8 2-digit Supplemental EAN/JAN-8 5-digit Supplemental UPC-E UPC-E 2digit Supplemental UPC-E 5digit Supplemental Standard Inverse Color Code 128 D014402 Barcode Samples (V4.2) Code Page 1 of 12 CortexDecoder™ Barcode Samples Underlined code names are enabled by default Standard Inverse Color Code 39 Checksum Full ASCII Standard Checksum Interleaved 2 of 5 Standard Mod16 Checksum 7DR Checksum Codabar (NW-7) By default, Start/Stop chars are displayed in output. Code 93 D014402 Barcode Samples (V4.2) Code Page 2 of 12 CortexDecoder™ Barcode Samples Underlined code names are enabled by default GS1 DataBar Omni/Truncated GS1 DataBar Stacked/Stacked Omni GS1 DataBar Limited GS1- GS1 DataBar Expanded GS1 DataBar Expanded Stacked DataBar The only difference between Omni(directional) and Truncated is that the bar height is taller for Omni and shorter for Truncated. GS1 DataBar Stacked implies it is truncated. EAN/JAN-8 with CC-A EAN/JAN-13 with CC-A 1234567021A12345678 3312345678903991234-abcd DataBar Limited with CC-B DataBar Limited with CC-A GS1- Composite 01131123456789061701061510A123456 010351234567890721abcdefghijklmnopqrstuv GS1-128 with CC-C GS1 DataBar and GS1-128 as part of the composite contains a link character indicating the existence of the composite code. EAN/JAN does not contain such a link. Therefore it is normal that the above EAN/JAN CCA samples may output the 1D only when the composite is not decodable. -

Image Pre-Processing to Improve Data Matrix Barcode Read Rates
University of New Hampshire University of New Hampshire Scholars' Repository Master's Theses and Capstones Student Scholarship Spring 2013 Image pre-processing to improve data matrix barcode read rates Nathan P. Brouwer University of New Hampshire, Durham Follow this and additional works at: https://scholars.unh.edu/thesis Recommended Citation Brouwer, Nathan P., "Image pre-processing to improve data matrix barcode read rates" (2013). Master's Theses and Capstones. 780. https://scholars.unh.edu/thesis/780 This Thesis is brought to you for free and open access by the Student Scholarship at University of New Hampshire Scholars' Repository. It has been accepted for inclusion in Master's Theses and Capstones by an authorized administrator of University of New Hampshire Scholars' Repository. For more information, please contact [email protected]. IMAGE PRE-PROCESSING TO IMPROVE DATA MATRIX BARCODE READ RATES BY NATHAN P. BROUWER B.S. Electrical Engineering 2011 University of New Hampshire THESIS Submitted to the University of New Hampshire in Partial Fulfillment of the Requirements for the Degree of Master of Science in Electrical Engineering M ay 2013 UMI Number: 1523784 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. Di!ss0?t&iori Publishing UMI 1523784 Published by ProQuest LLC 2013. Copyright in the Dissertation held by the Author. Microform Edition © ProQuest LLC. -

WDI4600 2D Barcode SCANNER
White Technologies Business Card Logo Wasp Logo Reversed Bee Only Regular Logo PMS116 CVC Regular Logo 4C Logo with Black Wasp WDI4600 2D BARCODE SCANNER Logo Outline Wasp Box Logo Productivity Solutions for Small Businesses The Wasp WDI4600 barcode scanner rapidly reads 1D and 2D barcodes, including QR codes. The WDI4600 streamlines data entry and allows you to instantly add barcode data to spreadsheets, word documents, and databases. Use the WDI4600 to improve efficiency and eliminate data-entry errors in healthcare, retail, office, shipping and receiving, travel, hospitality, manufacturing, and more. Installing and using the WDI4600 is fast and easy. Connect the WDI4600 directly to your PC’s USB port, and you’re ready to begin scanning 1D and 2D barcodes. A built-in, 4-Dot aiming guide sets a precise reading zone and reduces accidental reads. The laser’s center cross offers a targeted scanning when multiple barcodes are in view and allows for the reading of barcodes on mobile devices. The WDI4600 works with or without the use of a stand and automatically switches to the desired scanning mode. zx WASP WDI4600 FEATURES zx BENEFITS Read 1D barcodes up to 15” away and 2D barcodes up to 10” away Rapidly add 1D and 2D barcode data to documents, spreadsheets, and Withstands multiple 6’ drops to concrete databases Compatible with optional hands-free stand for autosense scanning Improve productivity and prevent data-entry errors Ergonomic design minimizes user fatigue Omnidirectional reading Reads all common 1D barcodes and 2D barcodes; including -

BCBP Implementation Guide Is Intended to Be Used As Guidance Material When Airlines Would Like to Implement Bar Coded Boarding Pass (BCBP)
BAR CODED BOARDING PASS (BCBP) IMPLEMENTATION GUIDE Effective Date: 1st June, 2018 Seven Edition Page | 1 DISCLAIMER. The information contained in this publication is subject to constant review in the light of changing government requirements and regulations. No reader should act on the basis of any such information without referring to applicable laws and regulations and/or without taking appropriate professional advice. Although every effort has been made to ensure accuracy, the International Air Transport Association shall not be held responsible for loss or damage caused by errors, omissions, misprints or misinterpretation of the contents hereof. Furthermore, the International Air Transport Association expressly disclaims all and any liability to any person, whether a purchaser of this publication or not, in respect of anything done or omitted, and the consequences of anything done or omitted, by any such person in reliance on the contents of this publication. No part of the Common Use Passenger Processing Systems Implementation Guide may be reproduced, recast, reformatted or transmitted in any form by any means, electronic or mechanical, including photocopying, recording or any information storage and retrieval system, without the prior written permission from: International Air Transport Association 800 Place Victoria, P.O. Box 113 Montreal, Quebec, Canada H4Z 1M1 © IATA 2017 Page | 2 TABLE OF CONTENTS 1. INTRODUCTION ..................................................................................... 6 1.1. Background .................................................................................................................. -

Guidelines for Bar Coding in the Pharmaceutical Supply Chain
HEALTHCARE DISTRIBUTION ALLIANCE Guidelines for Bar Coding in the Pharmaceutical Supply Chain Distributed by AmerisourceBergen Corporation with permission from HDA HDA GUIDELINES FOR BAR CODING IN THE PHARMACEUTICAL SUPPLY CHAIN HDA would like to thank Excellis Health Solutions LLC for their barcoding and serialization expertise in supporting the Bar Code Task Force development of the HDA Guidelines for Bar Coding in the Pharmaceutical Supply Chain. Excellis Health Solutions is a global provider of strategy and implementation consulting services within the life sciences and healthcare industries. Excellis provides deep subject matter expertise in compliance with global product traceability regulations, GS1 Standards and pharmaceutical/medical device supply chain systems implementation. Services include strategy, project/program management, comprehensive validation, change management, quality and regulatory compliance, managed services administration, software release management, subject matter support, global GS1/serialization/track-and-trace support; as well as education and training and systems integration. As a GS1 Solution Partner, Excellis Health Solutions has certified subject matter experts with GS1 Standards Professional Designation and GS1 Standards for UDI. Excellis Health Solutions, LLC 4 E. Bridge Street, Suite 300 New Hope, PA 18938 https://Excellishealth.com Contact: Gordon Glass, VP Consulting, at +1-609-847-9921 or [email protected] Revised November 2017 Although HDA has prepared or compiled the information presented herein in an effort to inform its members and the general public about the healthcare distribution industry, HDA does not warrant, either expressly or implicitly, the accuracy or completeness of this information and assumes no responsibility for its use. © Copyright 2017 Healthcare Distribution Alliance All rights reserved. -

GT2022 2D Imager Barcode Scanner Configuration Guide
GT2022 2D Imager Barcode Scanner Configuration Guide Table Of Contents Chapter 1 Getting Started .................................................................................................................................. 1 About This Guide ..................................................................................................................................... 1 Barcode Scanning ................................................................................................................................... 2 Barcode Programming ............................................................................................................................. 2 Factory Defaults ....................................................................................................................................... 3 Custom Defaults ...................................................................................................................................... 3 Chapter 2 Communication Interfaces .............................................................................................................. 4 Power-Saving Mode ................................................................................................................................ 4 TTL-232 Interface .................................................................................................................................... 5 Baud Rate ........................................................................................................................................ -

Understanding Verification Results
UNDERSTANDING VERIFICATION RESULTS A deeper look at what the software is telling you. 2019 1 © 2019 Cognex Confidential WHY AM I GETTING A NO DECODE WHEN THE CODE CAN BE READ USING ONE OF OUR READERS? 2 © 2019 Cognex Confidential ADDITIONAL REASONS FOR A NO DECODE . Are you using the correct aperture? . Are you using the right ISO Standard? . Are you using the right lighting angle? . Is the symbology enabled? . Is the camera in focus? . Is the code in the center of the FOV? . Is the code close to perpendicular? . Do the cell sizes look proportionate to one another? . Are the edges of the cells crisp? . Are all the components the finder pattern present? 3 © 2019 Cognex Confidential WHY WOULD MY GRADE FLUCTUATE FROM ONE LETTER TO ANOTHER? 4 © 2019 Cognex Confidential WHY AM I GETTING AN F? 12345678 5 © 2019 Cognex Confidential ISO STANDARD OVERVIEW 6 © 2019 Cognex Confidential BARCODE ISO STANDARDS These standards spell out the guidelines for creating, decoding, error correction, encodation, etc. BARCODE TYPE ISO STANDARD D ATA MATR IX ISO/IEC 16022 QR CODES ISO/IEC 18004 AZTEC ISO/IEC 24778 UPC/EAN ISO/IEC 15420 CODE 128 ISO/IEC 15417 CODE 39 ISO/IEC 16388 PDF 417 ISO/IEC 15438 7 © 2019 Cognex Confidential BARCODE QUALITY GRADING ISO STANDARDS Barcode Type 1 2 3 4 5 6 7 8 9 0 1D (Linear) 2D Marked Substrate Label Label Direct- Part-Mark (DPM) ISO IEC TR 29158 Standard ISO 15416 ISO 15415 (also called AIM-DPM) 8 © 2019 Cognex Confidential ISO/IEC 15415 (2D printed on flat labels) 9 © 2019 Cognex Confidential 29158 vs 15415? .