Frequency-Dependent Flight Activity in the Colour Polymorphic Wood Tiger Moth
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

33Rd ISCE, 2017 (GC/FT-IR Analysis of a Novel 2,4,6,9-Tetraene)
2017 ISCE/APACE Kyoto, Japan (August 24, 2017) GC / FT-IR analysis of a novel 2,4,6,9-tetraene occurring in a female pheromone gland of Parasemia plantaginis (Lepidoptera: Arctiidae) Yuta Muraki, Hideshi Naka, Atsushi Honma, Johanna Mappes, Kaisa Suisto, and Tetsu Ando* * Tokyo University of Agriculture and Technology, JapanJapan JICA, Can Tho University, Vietnam E-mail: [email protected] 3000 2000 1000 (cm-1) Representative lepidopteran sex pheromones Sex pheromones have been identified from more than 670 species. Male attractants have been reported for other 1300 species. Type I rice stem borer silkworm moth smaller tea tortrix Unsaturated fatty OH O H alcohols, acetates bombykol OAc O and aldehydes with H OA c a C10 –C18 chain Found most commonly (75%) Type II O O Polyunsaturated hydrocarbons and Z3,Z6,Z9-21:H epo3,Z6,Z9-19:H Z3,epo6,Z9-19:H their epoxides with a C17 –C23 chain Identified from evolved-insect groups (15%) plum cankerworm moth Milionia basalis giant geometrid moth Photos from http://www.jpmoth.org/ Phylogenetic tree of Lepidoptera yellow peach moth E10-16:Ald satin moth Z3,Z6,Z9-23:H plum cankerworm moth Z3,epo6,epo9-21:H Z3,Z6,Z9-21:H Z3,Z6,Z9-23:H Noctuoidea Bombycoidea Papillionoidea Geometroidea Sesioidea Pyraloidea Hesperioidea Pterophoroidea Zygaenoidea Gelechioidea Tortricoidea Yponomeutoidea Cossoidea Castnioidea Tineoidea Hepialoidea Incurvarioidea Nepticuloidea Ditrysia Eriocranioidea Monotrysia Zeugloptera Dacnonypha Type I pheromone Type II pheromone Paramecoptera Chemical structures of Type II pheromones Linoleic acid secretion O Z6,Z9-dienes OH E4,Z6,Z9-trienes mono- Z6,Z9,E11-trienes epoxides desaturation Z6,Z9,Z12-trienes Δ12 CnH2n+1 Δ4 Δ11 emerald moth secretion Linolenic acid O Z3,Z6,Z9-trienes OH 1,Z3,Z6,Z9-tetraenes mono- and Z3,Z6,Z9,E11-tetraenes di-epoxides desaturation Z3,Z6,Z9,Z11-tetraenes CnH2n+1 Δ1 Δ11 The known chemical diversity is still limited. -

Genetic and Phenotypic Differentiation As a Consequence of Host Plant Use by Lepidopteran Herbivores
ABSTRACT Title of dissertation: GENETIC AND PHENOTYPIC DIFFERENTIATION AS A CONSEQUENCE OF HOST PLANT USE BY LEPIDOPTERAN HERBIVORES J. Gwen Shlichta, Doctor in Philosophy, 2011 Dissertation directed by: Professor Pedro Barbosa, Department of Entomology In this dissertation, I focused on the role of plant hosts as a driving force leading to phenotypic and genotypic changes in insect herbivores. There are three main questions addressed: (1) Do generalist species’ populations have broad diet breadth or do they represent a mosaic of sub-populations, each having narrow diet breadths? (2) how does host plant affect the immune response of polyphagous herbivores, and (3) does host plant, or some aspect of host plant such as allelochemicals, alter the interaction between herbivore defense and parasitoid counter-defense? Do generalist species’ populations have broad diet breadth or do they represent a mosaic of sub-populations? In Chapter 1, I determined, using amplified fragment length polymorphisms (AFLPs), whether host plant-associated genetic differentiation (HAD) was exhibited by a suite of polyphagous tree feeding Lepidoptera. The objective of this research was to test HAD in a suite of polyphagous species that exhibit traits expected to be important in the formation of genetically divergent sub-populations. How does host plant affect the immune response of polyphagous species? In Chapter 2, the objective was to examine the effect of host plant species on the immune defenses of polyphagous lepidopteran herbivores, specifically the intensity of encapsulation measured as percent melanization, of three common forest Lepidoptera species. In Chapter 3, I discussed and assessed the potential role of immune responses in insect outbreaks. -

(Arctia Plantaginis) Through Trio Binning
bioRxiv preprint doi: https://doi.org/10.1101/2020.02.28.970020; this version posted March 2, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. A haplotype-resolved, de novo genome assembly for the wood tiger moth (Arctia plantaginis) through trio binning Eugenie C. Yen1*, Shane A. McCarthy2,3, Juan A. Galarza4, Tomas N. Generalovic1, Sarah Pelan3, Petr Nguyen5,6, Joana I. Meier1,7, Ian A. Warren1, Johanna Mappes4, Richard Durbin2,3 and Chris D. Jiggins1,7 1 Department of Zoology, University of Cambridge, Cambridge, CB2 3EJ, United Kingdom 2 Department of Genetics, University of Cambridge, Cambridge, CB2 3EH, United Kingdom 3 Wellcome Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridge, CB10 1SA, United Kingdom 4 Department of Biological and Environmental Science, University of Jyväskylä FI-40014, Jyväskylä, Finland 5 Biology Centre of the Czech Academy of Sciences, Institute of Entomology, 370 05 České Budějovice, Czech Republic 6 University of South Bohemia, Faculty of Science, 370 05 České Budějovice, Czech Republic 7 St John’s College, CB2 1TP, Cambridge, United Kingdom *Corresponding Author: Eugenie C. Yen. Department of Zoology, Downing Street, University of Cambridge, Cambridge, CB2 3EJ, UK. Email: [email protected]. Phone: +447402737277. 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.02.28.970020; this version posted March 2, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. -

Boloria Selene) on the Ochoco National Forest
2015 Surveys for Silver-bordered Fritillary (Boloria selene) On the Ochoco National Forest Prepared by Dana Ross (Entomologist, Lepidoptera Specialist) November 2015 0 SUMMARY The silver-bordered fritillary (Boloria selene) is designated as an ISSSSP Special Status/Sensitive Species in Oregon. In June and July of 2015, surveys were performed for this regionally rare butterfly on public lands managed by the Ochoco National Forest and Bureau of Land Management where there was either historical occurrence or a likelihood of undiscovered populations of the insect. Populations were documented at 8 distinct locations from Big Summit Prairie south to Williams and Gray prairies. All observed populations were in the North Fork Crooked River drainage at sites that contained at least some seasonally wet or marshy habitats with violets (the Boloria selene larval hostplant). Three fairly sizeable populations were located – at Elliot Creek (north Big Summit Prairie), along the BLM North Fork Crooked River/lower Lookout Creek site immediately south of Big Summit Prairie, and at NW Williams Prairie. Comparatively few of the butterflies were observed elsewhere. Observed damage to fragile critical habitats, such as at Elliot Creek (off-road vehicles) and Williams Prairie (cattle grazing), must be avoided in the future to prevent habitat degradation, population decline and potential local extirpation of the insect. It is recommended that additional surveys for silver-bordered fritillary take place on National Forest and BLM lands to: 1)more completely document the species regional range and distribution; 2) to more accurately assess population/meta-population size; 3) to assess the impact to future generations from observed mid-season damage to critical habitats; 4) to verify benefits (increased abundance or local persistence) to populations from habitat protection or habitat restoration; 5) to determine butterfly response to short or long term environmental conditions such as drought. -

Sustainable Farming of the Mealworm Tenebrio Molitor for the Production of Food and Feed
Z. Naturforsch. 2017; 72(9–10)c: 337–349 Thorben Grau, Andreas Vilcinskas and Gerrit Joop* Sustainable farming of the mealworm Tenebrio molitor for the production of food and feed DOI 10.1515/znc-2017-0033 cost is expected to increase [5]. Alternatives are there- Received March 3, 2017; revised March 27, 2017; accepted April 11, fore required to reduce the EU’s economic dependence 2017 on imports [6]. Furthermore, large amounts of food and nonprofitable side streams from industrial processes Abstract: The farming of edible insects is an alternative are currently wasted [7], but this could be used as feed strategy for the production of protein-rich food and feed for insects, which can convert diverse waste streams with a low ecological footprint. The industrial produc- into protein. Edible insects are therefore gaining atten- tion of insect-derived protein is more cost-effective and tion among the research community: in 2007, the search energy-efficient than livestock farming or aquaculture. term “edible insects” recovered 12 publications listed in The mealworm Tenebrio molitor is economically among PubMed, but this had increased to more than 40 publi- the most important species used for the large-scale con- cations in 2016. More than 2000 edible insect species version of plant biomass into protein. Here, we review the are known worldwide, but only a few are produced com- mass rearing of this species and its conversion into food mercially [8, 9]. These species show diverse nutritional and feed, focusing on challenges such as the contamina- profiles but insects are generally considered as good tion of food/feed products with bacteria from the insect alternative protein sources for humans, livestock and gut and the risk of rapidly spreading pathogens and para- aquaculture, which can be produced in an environmen- sites. -

Tarset and Greystead Biological Records
Tarset and Greystead Biological Records published by the Tarset Archive Group 2015 Foreword Tarset Archive Group is delighted to be able to present this consolidation of biological records held, for easy reference by anyone interested in our part of Northumberland. It is a parallel publication to the Archaeological and Historical Sites Atlas we first published in 2006, and the more recent Gazeteer which both augments the Atlas and catalogues each site in greater detail. Both sets of data are also being mapped onto GIS. We would like to thank everyone who has helped with and supported this project - in particular Neville Geddes, Planning and Environment manager, North England Forestry Commission, for his invaluable advice and generous guidance with the GIS mapping, as well as for giving us information about the archaeological sites in the forested areas for our Atlas revisions; Northumberland National Park and Tarset 2050 CIC for their all-important funding support, and of course Bill Burlton, who after years of sharing his expertise on our wildflower and tree projects and validating our work, agreed to take this commission and pull everything together, obtaining the use of ERIC’s data from which to select the records relevant to Tarset and Greystead. Even as we write we are aware that new records are being collected and sites confirmed, and that it is in the nature of these publications that they are out of date by the time you read them. But there is also value in taking snapshots of what is known at a particular point in time, without which we have no way of measuring change or recognising the hugely rich biodiversity of where we are fortunate enough to live. -

FAUNA Vernacular Name FAUNA Scientific Name Read More
FAUNA Vernacular Name FAUNA Scientific Name Read more a European Hoverfly Pocota personata https://www.naturespot.org.uk/species/pocota-personata a small black wasp Stigmus pendulus https://www.bwars.com/wasp/crabronidae/pemphredoninae/stigmus-pendulus a spider-hunting wasp Anoplius concinnus https://www.bwars.com/wasp/pompilidae/pompilinae/anoplius-concinnus a spider-hunting wasp Anoplius nigerrimus https://www.bwars.com/wasp/pompilidae/pompilinae/anoplius-nigerrimus Adder Vipera berus https://www.woodlandtrust.org.uk/trees-woods-and-wildlife/animals/reptiles-and-amphibians/adder/ Alga Cladophora glomerata https://en.wikipedia.org/wiki/Cladophora Alga Closterium acerosum https://www.algaebase.org/search/species/detail/?species_id=x44d373af81fe4f72 Alga Closterium ehrenbergii https://www.algaebase.org/search/species/detail/?species_id=28183 Alga Closterium moniliferum https://www.algaebase.org/search/species/detail/?species_id=28227&sk=0&from=results Alga Coelastrum microporum https://www.algaebase.org/search/species/detail/?species_id=27402 Alga Cosmarium botrytis https://www.algaebase.org/search/species/detail/?species_id=28326 Alga Lemanea fluviatilis https://www.algaebase.org/search/species/detail/?species_id=32651&sk=0&from=results Alga Pediastrum boryanum https://www.algaebase.org/search/species/detail/?species_id=27507 Alga Stigeoclonium tenue https://www.algaebase.org/search/species/detail/?species_id=60904 Alga Ulothrix zonata https://www.algaebase.org/search/species/detail/?species_id=562 Algae Synedra tenera https://www.algaebase.org/search/species/detail/?species_id=34482 -

Comparative Transcriptomics of Albino and Warningly Coloured Caterpillars
bioRxiv preprint doi: https://doi.org/10.1101/336636; this version posted June 2, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1! Comparative transcriptomics of albino and warningly coloured caterpillars 2! Juan A. Galarza§. 3! §Centre of Excellence in Biological Interactions. University of Jyväskylä, Finland. 4! e-mail: [email protected] 5! 6! Data deposition. All raw reads and assemblies can be found at National Center for 7! Biotechnology Information (NCBI) under project number PRJNA449279 with accession 8! GGLW00000000. 9! 10! Abstract: 11! 12! Colouration is perhaps one of the most prominent adaptations for survival and reproduction 13! of most taxa. Colouration is of particular importance for aposematic species, which rely on 14! their colouring and patterning to act as a warning signal against predators. Most research has 15! focused on the evolution of warning colouration by natural selection. However, little 16! information is available for colour mutants of aposematic species, particularly at the genomic 17! level. Here I compare the transcriptomes of albino mutant caterpillars of the wood tiger moth 18! (Arctia plantaginis) to those of their full-sibs having their distinctive orange-black warning 19! colouration. The results showed >300 differentially expressed genes transcriptome-wide. 20! Genes involved in the immune system, structural constituents of cuticle, and peptidase 21! activity were mostly down-regulated in the albino larvae. Surprisingly, higher expression was 22! observed in core melanin genes from albino larvae, suggesting that melanin synthesis may be 23! disrupted in terminal ends of the pathway during its final conversion. -

Geographic Mosaic of Selection by Avian Predators on Hindwing Warning Colour in a Polymorphic Aposematic Moth
UvA-DARE (Digital Academic Repository) Geographic mosaic of selection by avian predators on hindwing warning colour in a polymorphic aposematic moth Rönkä, K.; Valkonen, J.K.; Nokelainen, O.; Rojas, B.; Gordon, S.; Burdfield-Steel, E.; Mappes, J. DOI 10.1111/ele.13597 Publication date 2020 Document Version Final published version Published in Ecology Letters License CC BY Link to publication Citation for published version (APA): Rönkä, K., Valkonen, J. K., Nokelainen, O., Rojas, B., Gordon, S., Burdfield-Steel, E., & Mappes, J. (2020). Geographic mosaic of selection by avian predators on hindwing warning colour in a polymorphic aposematic moth. Ecology Letters, 23(11), 1654-1663. https://doi.org/10.1111/ele.13597 General rights It is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), other than for strictly personal, individual use, unless the work is under an open content license (like Creative Commons). Disclaimer/Complaints regulations If you believe that digital publication of certain material infringes any of your rights or (privacy) interests, please let the Library know, stating your reasons. In case of a legitimate complaint, the Library will make the material inaccessible and/or remove it from the website. Please Ask the Library: https://uba.uva.nl/en/contact, or a letter to: Library of the University of Amsterdam, Secretariat, Singel 425, 1012 WP Amsterdam, The Netherlands. You will be contacted as soon as possible. UvA-DARE is a service provided by the library of the University of Amsterdam (https://dare.uva.nl) Download date:24 Sep 2021 Ecology Letters, (2020) 23: 1654–1663 doi: 10.1111/ele.13597 LETTER Geographic mosaic of selection by avian predators on hindwing warning colour in a polymorphic aposematic moth Abstract Katja Ronk€ a,€ 1,2,3* Warning signals are predicted to develop signal monomorphism via positive frequency-dependent Janne K. -
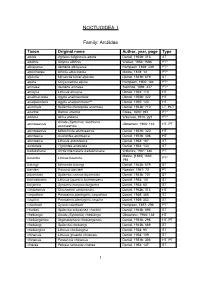
Noctuoidea I
NOCTUOIDEA I Family: Arctiidae Taxon Original name Author, year, page Type albida Agrisius fuliginosus albida Daniel, 1952b: 316 ST albifinis Sidyma albifinis Walker, 1856: 1686 PT* albisparsa Aemene albisparsa Hampson, 1898: 439 PT* albocinerea Ghoria albocinerea Moore, 1878: 13 PT* alpicola Micrarctia hönei alpicola Daniel, 1943b: 679 ST alpina Chrysorabdia alpina Hampson, 1900: 184 PT* amnaea Aemene amnaea Swinhoe, 1894: 437 PT* amoyca Lithosia amoyca Daniel, 1954: 110 HT analimaculata Agylla analimaculata Daniel, 1952b: 322 HT analipunctaria Agylla analipunctaria*** Daniel, 1955: 143 HT anormala Spilarctia rhodophila anormala Daniel, 1943b: 710 LT, PLT ariadne Bizone ariadne Elwes, 1890: 394 PT* arizana Ilema arizana Wileman, 1910: 221 PT* Amata (Syntomis) xanthoma atuntseensis Obraztsov, 1966: 146 HT, PT atuntseensis atuntseensis Miltochrista atuntseensis Daniel, 1951b: 320 HT atuntseica Asurioides atuntseica Daniel, 1951b: 308 HT atuntseica Lithosia antuntseica Daniel, 1954: 107 ST aureolata Tigrioides aureolata Daniel, 1954: 133 ST badakshana Arctia intercalaris badakhshana Wiltshire, 1961: 340 PT Moore, [1866] 1865: basinota Lihosia basinota PT* 798 batangi Micrarctia batangi Daniel, 1943b: 679 ST benderi Paraona benderi Roesler, 1967: 72 PT bipunctata Spilarctia comma bipunctata Daniel, 1943b: 701 ST brunnescens Lithosia japonica brunnescens Daniel, 1954: 101 ST bulgarica Syntomis marjana bulgarica Daniel, 1934: 60 ST cantonensis Chionaema cantonensis Daniel, 1952b: 313 HT carpathica Parasemia plantaginis carpathica Daniel, 1939: -

Toxicity and Mode of Action of Steroid and Terpenoid Secondary Plant Metabolites Against Economically Important Pest Insects in Agriculture
Faculty of Bioscience Engineering Academic year 2011-2012 TOXICITY AND MODE OF ACTION OF STEROID AND TERPENOID SECONDARY PLANT METABOLITES AGAINST ECONOMICALLY IMPORTANT PEST INSECTS IN AGRICULTURE Lic. Ellen DE GEYTER Thesis submitted in fulfilment of the requirements for the degree of Doctor (Ph.D.) in Applied Biological Sciences Promotors: Prof. dr. ir. Guy Smagghe Department of Crop Protection Faculty of Bioscience Engineering Ghent University Prof. dr. Danny Geelen Department of Plant Production Faculty of Bioscience Engineering Ghent University Dean: Prof. dr. ir. Guido Van Huylenbroeck Rector: Prof. dr. Paul Van Cauwenberge Dutch title: Toxiciteit en werkingswijze van steroïde en terpenoïde secundaire plantmetabolieten bij economisch belangrijke schadelijke insecten in de landbouw Cite as: De Geyter, E., 2012. Toxicity and mode of action of steroid and terpenoid secondary plant metabolites against economically important pest insects in agriculture. PhD dissertation, Faculty of Bioscience Engineering, Ghent University, Ghent. ISBN: 978-90-5989-536-2 The author and promotors give the authorization to consult and to copy parts of this work for personal use only. Every other use is subject to the copyright laws. Permission to reproduce any material contained in this work should be obtained from the author. This research was supported by a fellowship of the Special Research Fund of Ghent University (BOF-UGent). EXAMINATION COMMITTEE Promotors: Prof. dr. ir. Guy Smagghe Department of Crop Protection Faculty of Bioscience Engineering Ghent University Prof. dr. Danny Geelen Department of Plant Production Faculty of Bioscience Engineering Ghent University Members: Prof. dr. ir. Stefaan De Smet (chairman) Department of Animal Production Faculty of Bioscience Engineering Ghent University Prof. -

No Evidence of Quantitative Signal Honesty Across Species of Aposematic
1 No evidence of quantitative signal honesty across species of aposematic 2 burnet moths (Lepidoptera: Zygaenidae) 3 Running title: Testing signal honesty across burnet moths 4 Authors: Emmanuelle Sophie Briolat1,*, Mika Zagrobelny2, Carl Erik Olsen2, Jonathan D. Blount1 and 5 Martin Stevens1 6 1: Centre for Ecology & Conservation, College of Life & Environmental Sciences, University of Exeter, Penryn 7 Campus, Penryn, Cornwall, TR10 9FE, UK. 8 2: Plant Biochemistry Laboratory and Copenhagen Plant Science Centre, Department of Plant and Environmental 9 Sciences, University of Copenhagen, 40 Thorvaldsensvej, DK-1871 Frederiksberg C, Copenhagen, Denmark 10 *: Corresponding author email: [email protected] 11 Tel.: (+44)1326 619784 12 Fax: NA 13 14 15 Acknowledgments 16 The authors were funded by the BBSRC (SWBio DTP studentship, ref. 1355867) and Danish Council 17 for Independent Research (DFF–1323-00088). We would like to thank Alain Bourgon, Helen and 18 Christian Briolat, David Demergès, Pierre Desriaux, Claude Dutreix, Anne and Denis Filosa, Alain 19 Migeon, Marc Nicolle, Andrew Szopa-Comley, W. G. Tremewan and in particular Eric Drouet, for 20 assistance with specimen collection, as well as Thomas Currie, Sarah C. Paul and Jolyon Troscianko 21 for helpful discussion. 22 23 Conflict of Interest 24 All the authors of this work declare no conflict of interest. 25 26 27 1 28 Abstract 29 Many defended species use conspicuous visual warning signals to deter potential predators 30 from attacking. Traditional theory holds that these signals should converge on similar forms, 31 yet variation in visual traits and the levels of defensive chemicals is common, both within and 32 between species.