33Rd ISCE, 2017 (GC/FT-IR Analysis of a Novel 2,4,6,9-Tetraene)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Frequency-Dependent Flight Activity in the Colour Polymorphic Wood Tiger Moth
Current Zoology 61 (4): 765–772, 2015 Frequency-dependent flight activity in the colour polymorphic wood tiger moth †* † Bibiana ROJAS , Swanne P. GORDON , Johanna MAPPES University of Jyvaskyla, Centre of Excellence in Biological Interactions, Department of Biology and Environmental Sciences, PO Box 35, FI 40001, Finland Abstract Predators efficiently learn to avoid one type of warning signal rather than several, making colour polymorphisms un- expected. Aposematic wood tiger moth males Parasemia plantaginis have either white or yellow hindwing coloration across Eu- rope. Previous studies indicate that yellow males are better defended from predators, while white males have a positively fre- quency-dependent mating advantage. However, the potential frequency-dependent behavioural differences in flight between the morphs, as well as the role of male-male interactions in inducing flying activity, have not been previously considered. We ran an outdoor cage experiment where proportions of both male morphs were manipulated to test whether flying activity was frequency- dependent and differed between morphs. The white morph was significantly more active than the yellow one across all treatments, and sustained activity for longer. Overall activity for both morphs was considerably lower in the yellow-biased environment, suggesting that higher proportions of yellow males in a population may lead to overall reduced flying activity. The activity of the yellow morph also followed a steeper, narrower curve than that of the white morph during peak female calling activity. We sug- gest that white males, with their presumably less costly defences, have more resources to invest in flight for predator escape and finding mates. Yellow males, which are better protected but less sexually selected, may instead compensate their lower flight ac- tivity by ‘flying smart’ during the peak female-calling periods. -

(Arctia Plantaginis) Through Trio Binning
bioRxiv preprint doi: https://doi.org/10.1101/2020.02.28.970020; this version posted March 2, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. A haplotype-resolved, de novo genome assembly for the wood tiger moth (Arctia plantaginis) through trio binning Eugenie C. Yen1*, Shane A. McCarthy2,3, Juan A. Galarza4, Tomas N. Generalovic1, Sarah Pelan3, Petr Nguyen5,6, Joana I. Meier1,7, Ian A. Warren1, Johanna Mappes4, Richard Durbin2,3 and Chris D. Jiggins1,7 1 Department of Zoology, University of Cambridge, Cambridge, CB2 3EJ, United Kingdom 2 Department of Genetics, University of Cambridge, Cambridge, CB2 3EH, United Kingdom 3 Wellcome Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridge, CB10 1SA, United Kingdom 4 Department of Biological and Environmental Science, University of Jyväskylä FI-40014, Jyväskylä, Finland 5 Biology Centre of the Czech Academy of Sciences, Institute of Entomology, 370 05 České Budějovice, Czech Republic 6 University of South Bohemia, Faculty of Science, 370 05 České Budějovice, Czech Republic 7 St John’s College, CB2 1TP, Cambridge, United Kingdom *Corresponding Author: Eugenie C. Yen. Department of Zoology, Downing Street, University of Cambridge, Cambridge, CB2 3EJ, UK. Email: [email protected]. Phone: +447402737277. 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.02.28.970020; this version posted March 2, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. -

Boloria Selene) on the Ochoco National Forest
2015 Surveys for Silver-bordered Fritillary (Boloria selene) On the Ochoco National Forest Prepared by Dana Ross (Entomologist, Lepidoptera Specialist) November 2015 0 SUMMARY The silver-bordered fritillary (Boloria selene) is designated as an ISSSSP Special Status/Sensitive Species in Oregon. In June and July of 2015, surveys were performed for this regionally rare butterfly on public lands managed by the Ochoco National Forest and Bureau of Land Management where there was either historical occurrence or a likelihood of undiscovered populations of the insect. Populations were documented at 8 distinct locations from Big Summit Prairie south to Williams and Gray prairies. All observed populations were in the North Fork Crooked River drainage at sites that contained at least some seasonally wet or marshy habitats with violets (the Boloria selene larval hostplant). Three fairly sizeable populations were located – at Elliot Creek (north Big Summit Prairie), along the BLM North Fork Crooked River/lower Lookout Creek site immediately south of Big Summit Prairie, and at NW Williams Prairie. Comparatively few of the butterflies were observed elsewhere. Observed damage to fragile critical habitats, such as at Elliot Creek (off-road vehicles) and Williams Prairie (cattle grazing), must be avoided in the future to prevent habitat degradation, population decline and potential local extirpation of the insect. It is recommended that additional surveys for silver-bordered fritillary take place on National Forest and BLM lands to: 1)more completely document the species regional range and distribution; 2) to more accurately assess population/meta-population size; 3) to assess the impact to future generations from observed mid-season damage to critical habitats; 4) to verify benefits (increased abundance or local persistence) to populations from habitat protection or habitat restoration; 5) to determine butterfly response to short or long term environmental conditions such as drought. -

Sustainable Farming of the Mealworm Tenebrio Molitor for the Production of Food and Feed
Z. Naturforsch. 2017; 72(9–10)c: 337–349 Thorben Grau, Andreas Vilcinskas and Gerrit Joop* Sustainable farming of the mealworm Tenebrio molitor for the production of food and feed DOI 10.1515/znc-2017-0033 cost is expected to increase [5]. Alternatives are there- Received March 3, 2017; revised March 27, 2017; accepted April 11, fore required to reduce the EU’s economic dependence 2017 on imports [6]. Furthermore, large amounts of food and nonprofitable side streams from industrial processes Abstract: The farming of edible insects is an alternative are currently wasted [7], but this could be used as feed strategy for the production of protein-rich food and feed for insects, which can convert diverse waste streams with a low ecological footprint. The industrial produc- into protein. Edible insects are therefore gaining atten- tion of insect-derived protein is more cost-effective and tion among the research community: in 2007, the search energy-efficient than livestock farming or aquaculture. term “edible insects” recovered 12 publications listed in The mealworm Tenebrio molitor is economically among PubMed, but this had increased to more than 40 publi- the most important species used for the large-scale con- cations in 2016. More than 2000 edible insect species version of plant biomass into protein. Here, we review the are known worldwide, but only a few are produced com- mass rearing of this species and its conversion into food mercially [8, 9]. These species show diverse nutritional and feed, focusing on challenges such as the contamina- profiles but insects are generally considered as good tion of food/feed products with bacteria from the insect alternative protein sources for humans, livestock and gut and the risk of rapidly spreading pathogens and para- aquaculture, which can be produced in an environmen- sites. -

Geographic Mosaic of Selection by Avian Predators on Hindwing Warning Colour in a Polymorphic Aposematic Moth
UvA-DARE (Digital Academic Repository) Geographic mosaic of selection by avian predators on hindwing warning colour in a polymorphic aposematic moth Rönkä, K.; Valkonen, J.K.; Nokelainen, O.; Rojas, B.; Gordon, S.; Burdfield-Steel, E.; Mappes, J. DOI 10.1111/ele.13597 Publication date 2020 Document Version Final published version Published in Ecology Letters License CC BY Link to publication Citation for published version (APA): Rönkä, K., Valkonen, J. K., Nokelainen, O., Rojas, B., Gordon, S., Burdfield-Steel, E., & Mappes, J. (2020). Geographic mosaic of selection by avian predators on hindwing warning colour in a polymorphic aposematic moth. Ecology Letters, 23(11), 1654-1663. https://doi.org/10.1111/ele.13597 General rights It is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), other than for strictly personal, individual use, unless the work is under an open content license (like Creative Commons). Disclaimer/Complaints regulations If you believe that digital publication of certain material infringes any of your rights or (privacy) interests, please let the Library know, stating your reasons. In case of a legitimate complaint, the Library will make the material inaccessible and/or remove it from the website. Please Ask the Library: https://uba.uva.nl/en/contact, or a letter to: Library of the University of Amsterdam, Secretariat, Singel 425, 1012 WP Amsterdam, The Netherlands. You will be contacted as soon as possible. UvA-DARE is a service provided by the library of the University of Amsterdam (https://dare.uva.nl) Download date:24 Sep 2021 Ecology Letters, (2020) 23: 1654–1663 doi: 10.1111/ele.13597 LETTER Geographic mosaic of selection by avian predators on hindwing warning colour in a polymorphic aposematic moth Abstract Katja Ronk€ a,€ 1,2,3* Warning signals are predicted to develop signal monomorphism via positive frequency-dependent Janne K. -
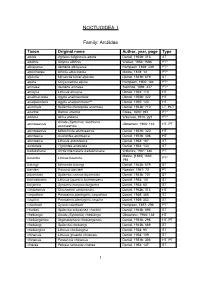
Noctuoidea I
NOCTUOIDEA I Family: Arctiidae Taxon Original name Author, year, page Type albida Agrisius fuliginosus albida Daniel, 1952b: 316 ST albifinis Sidyma albifinis Walker, 1856: 1686 PT* albisparsa Aemene albisparsa Hampson, 1898: 439 PT* albocinerea Ghoria albocinerea Moore, 1878: 13 PT* alpicola Micrarctia hönei alpicola Daniel, 1943b: 679 ST alpina Chrysorabdia alpina Hampson, 1900: 184 PT* amnaea Aemene amnaea Swinhoe, 1894: 437 PT* amoyca Lithosia amoyca Daniel, 1954: 110 HT analimaculata Agylla analimaculata Daniel, 1952b: 322 HT analipunctaria Agylla analipunctaria*** Daniel, 1955: 143 HT anormala Spilarctia rhodophila anormala Daniel, 1943b: 710 LT, PLT ariadne Bizone ariadne Elwes, 1890: 394 PT* arizana Ilema arizana Wileman, 1910: 221 PT* Amata (Syntomis) xanthoma atuntseensis Obraztsov, 1966: 146 HT, PT atuntseensis atuntseensis Miltochrista atuntseensis Daniel, 1951b: 320 HT atuntseica Asurioides atuntseica Daniel, 1951b: 308 HT atuntseica Lithosia antuntseica Daniel, 1954: 107 ST aureolata Tigrioides aureolata Daniel, 1954: 133 ST badakshana Arctia intercalaris badakhshana Wiltshire, 1961: 340 PT Moore, [1866] 1865: basinota Lihosia basinota PT* 798 batangi Micrarctia batangi Daniel, 1943b: 679 ST benderi Paraona benderi Roesler, 1967: 72 PT bipunctata Spilarctia comma bipunctata Daniel, 1943b: 701 ST brunnescens Lithosia japonica brunnescens Daniel, 1954: 101 ST bulgarica Syntomis marjana bulgarica Daniel, 1934: 60 ST cantonensis Chionaema cantonensis Daniel, 1952b: 313 HT carpathica Parasemia plantaginis carpathica Daniel, 1939: -

Toxicity and Mode of Action of Steroid and Terpenoid Secondary Plant Metabolites Against Economically Important Pest Insects in Agriculture
Faculty of Bioscience Engineering Academic year 2011-2012 TOXICITY AND MODE OF ACTION OF STEROID AND TERPENOID SECONDARY PLANT METABOLITES AGAINST ECONOMICALLY IMPORTANT PEST INSECTS IN AGRICULTURE Lic. Ellen DE GEYTER Thesis submitted in fulfilment of the requirements for the degree of Doctor (Ph.D.) in Applied Biological Sciences Promotors: Prof. dr. ir. Guy Smagghe Department of Crop Protection Faculty of Bioscience Engineering Ghent University Prof. dr. Danny Geelen Department of Plant Production Faculty of Bioscience Engineering Ghent University Dean: Prof. dr. ir. Guido Van Huylenbroeck Rector: Prof. dr. Paul Van Cauwenberge Dutch title: Toxiciteit en werkingswijze van steroïde en terpenoïde secundaire plantmetabolieten bij economisch belangrijke schadelijke insecten in de landbouw Cite as: De Geyter, E., 2012. Toxicity and mode of action of steroid and terpenoid secondary plant metabolites against economically important pest insects in agriculture. PhD dissertation, Faculty of Bioscience Engineering, Ghent University, Ghent. ISBN: 978-90-5989-536-2 The author and promotors give the authorization to consult and to copy parts of this work for personal use only. Every other use is subject to the copyright laws. Permission to reproduce any material contained in this work should be obtained from the author. This research was supported by a fellowship of the Special Research Fund of Ghent University (BOF-UGent). EXAMINATION COMMITTEE Promotors: Prof. dr. ir. Guy Smagghe Department of Crop Protection Faculty of Bioscience Engineering Ghent University Prof. dr. Danny Geelen Department of Plant Production Faculty of Bioscience Engineering Ghent University Members: Prof. dr. ir. Stefaan De Smet (chairman) Department of Animal Production Faculty of Bioscience Engineering Ghent University Prof. -

Checklists of Protected and Threatened Species in Ireland. Irish Wildlife Manuals, No
ISSN 1393 – 6670 N A T I O N A L P A R K S A N D W I L D L I F E S ERVICE CHECKLISTS OF PROTECTED AND THREATENED SPECIES IN IRELAND Brian Nelson, Sinéad Cummins, Loraine Fay, Rebecca Jeffrey, Seán Kelly, Naomi Kingston, Neil Lockhart, Ferdia Marnell, David Tierney and Mike Wyse Jackson I R I S H W I L D L I F E M ANUAL S 116 National Parks and Wildlife Service (NPWS) commissions a range of reports from external contractors to provide scientific evidence and advice to assist it in its duties. The Irish Wildlife Manuals series serves as a record of work carried out or commissioned by NPWS, and is one means by which it disseminates scientific information. Others include scientific publications in peer reviewed journals. The views and recommendations presented in this report are not necessarily those of NPWS and should, therefore, not be attributed to NPWS. Front cover, small photographs from top row: Coastal heath, Howth Head, Co. Dublin, Maurice Eakin; Red Squirrel Sciurus vulgaris, Eddie Dunne, NPWS Image Library; Marsh Fritillary Euphydryas aurinia, Brian Nelson; Puffin Fratercula arctica, Mike Brown, NPWS Image Library; Long Range and Upper Lake, Killarney National Park, NPWS Image Library; Limestone pavement, Bricklieve Mountains, Co. Sligo, Andy Bleasdale; Meadow Saffron Colchicum autumnale, Lorcan Scott; Barn Owl Tyto alba, Mike Brown, NPWS Image Library; A deep water fly trap anemone Phelliactis sp., Yvonne Leahy; Violet Crystalwort Riccia huebeneriana, Robert Thompson Main photograph: Short-beaked Common Dolphin Delphinus delphis, -

Systematic List of the Noctuoidea of Europe (Notodontidae, Nolidae, Arctiidae, Lymantriidae, Erebidae, Micronoctuidae, and Noctuidae)
Esperiana Buchreihe zur Entomologie Bd. 11: 93-205 Schwanfeld, 29. Juni 2005 ISBN 3-938249-01-3 Systematic List of the Noctuoidea of Europe (Notodontidae, Nolidae, Arctiidae, Lymantriidae, Erebidae, Micronoctuidae, and Noctuidae) Michael FIBIGER and Hermann H. HACKER Superfamily NOCTUOIDEA LATREILLE, 1809 Remarks to Classification In the European List of Noctuidae (FIBIGER and HACKER, 1991) we refrained from writing a justification for subdividing the family into subfamilies and tribes. Our understanding of the classification of the family has progressed since then, but only to some extent therefore several paraphyletic or even polyphyletic groupings still remain in the Noctuidae (s.l.) and in the other families dealt with here. Most of the tribes listed here are monophyletic and we believe that most of them will stand the test of time. In groups where research is lasting, we refer to the latest knowledge available. For example the family Noctuidae has now been divided into three families, the Noctuidae, Micronoctuidae and Erebidae, these corresponding roughly to the groups previously called “quadrifid“ and “trifid“ noctuids. Ongoing research, however, suggests that the arctiid clade might be derived from within the Erebidae (WELLER and MITCHELL, pers. com.). Other important results have been published in the last 13 years, some of which are: KITCHING and RAWLINS (in KRISTENSEN, 1998); KITCHING and YELA (1999); SPEIDEL, FÄNGER and NAUMANN (1996); SPEIDEL and NAUMANN (1996); POOLE (1995, and his catalogue from 1989); the North American Moths of North America (MONA) book series: LAFONTAINE and POOLE (1991), POOLE (1995), LAFONTAINE (1998, 2004); BECK (1996, 1999, 2000); the volumes of the book series Noctuidae Europaeae (1990-2003); and many papers in Esperiana. -

Butterflies and Moths of the Yukon
Butterflies and moths of the Yukon FRONTISPIECE. Some characteristic arctic and alpine butterflies and moths from the Yukon. Upper, males of the nymphalid butterflies Oeneis alpina Kurentzov (left) and Boloria natazhati (Gibson) (right), normally encountered on rocky tundra slopes; Middle, males of the alpine arctiid moths Pararctia yarrowi (Stretch) (left), typically on dry rocky slopes with willow, and Acsala anomala Benjamin (right), confined to the Yukon and Alaska and shown here on the characteristic dry rocky habitat of the lichen-feeding larvae; Lower, (left) female of the arctiid moth Dodia kononenkoi Chistyakov and Lafontaine from dry rocky tundra slopes, and (right) a mated pair of the noctuid moth Xestia aequeva (Benjamin), showing the reduced wings of the female. All species were photographed at Windy Pass, Ogilvie Mountains (see book frontispiece), except for B. natazhati (Richardson Mountains). Forewing length of these species is about 2 cm (first 3 species) and 1.5 cm (last 3). 723 Butterflies and Moths (Lepidoptera) of the Yukon J.D. LAFONTAINE and D.M. WOOD Biological Resources Program, Research Branch, Agriculture and Agri-Food Canada K.W. Neatby Bldg., Ottawa, Ontario, Canada K1A 0C6 Abstract. An annotated list of the 518 species of Lepidoptera known from the Yukon is presented with a zoogeographic analysis of the fauna. Topics discussed are: historical review of Yukon collecting and research; the expected size of the Yukon fauna (about 2000 species); zoogeographic affinities; special features of Yukon fauna (endemic species, disjunct species, biennialism, flightless species). There are 191 species of Lepidoptera (37% of the fauna) in the Yukon that occur in both Nearctic and Palaearctic regions. -
Ireland Red List No. 9: Macro-Moths (Lepidoptera)
Ireland Red List No. 9 Macro-moths (Lepidoptera) Ireland Red List No. 9 Macro-moths (Lepidoptera) D. Allen1, M. O’Donnell2, B. Nelson3, A. Tyner4, K.G.M. Bond5, T. Bryant6, A. Crory7, C. Mellon1, J. O’Boyle8, E. O’Donnell9, T. Rolston10, R. Sheppard11, P. Strickland12, U. Fitzpatrick13, E. Regan14. 1Allen & Mellon Environmental Ltd, 21A Windor Avenue, Belfast, BT9 6EE 2Joffre Rose, Clone, Castletown, Gorey, Co. Wexford 3National Parks & Wildlife Service, Department of the Arts, Heritage and the Gaeltacht, Ely Place, Dublin D02 TW98 4Honeyoak, Cronykeery, Ashford, Co. Wicklow 5Zoology, Ecology and Plant Science, Distillery Fields, North Mall, University College Cork 6Knocknarea, Priest’s Road, Tramore, Co. Waterford 7113 Dundrum Road, Newcastle, Co. Down, BT33 0LN 8Natural Environment Division, Northern Ireland Environment Agency, Department of Agriculture, Environment and Rural Affairs, Klondyke Building, Cromac Avenue, Belfast, BT7 2JA 95 Forgehill Rise, Stamullen, Co. Meath 1042 Beechdene Gardens, Lisburn, Co. Antrim, BT28 3JH 11Carnowen, Raphoe, Co. Donegal 1222 Newtown Court, Maynooth, Co. Kildare 13National Biodiversity Data Centre, WIT west campus, Carriganore, Waterford 14The Biodiversity Consultancy, 3E King’s Parade, Cambridge, CB2 1SJ Citation: Allen, D., O’Donnell, M., Nelson, B., Tyner, A., Bond, K.G.M., Bryant, T., Crory, A., Mellon, C., O’Boyle, J., O’Donnell, E., Rolston, T., Sheppard, R., Strickland, P., Fitzpatrick, U., & Regan, E. (2016) Ireland Red List No. 9: Macro-moths (Lepidoptera). National Parks and Wildlife Service, Department of Arts, Heritage and the Gaeltacht, Dublin, Ireland. Cover photos: Bottom left to top right: White Prominent Leucodonta bicoloria—photo: Brian Nelson; Burren Green Calamia tridens—photo: Brian Nelson; Figure of Eight Diloba caeruleocephala caterpillar—photo: Geoff Campbell; Thrift Clearwing Pyropteron muscaeformis— photo: Eamonn O’Donnell; Yellow Shell Camptogramma bilineata—photo: Geoff Campbell. -

Lepidoptera Conservation Bulletin No. 11 (April 2010-March 2011)
Lepidoptera Conservation Bulletin Number 11 April 2010 – March 2011 Butterfly Conservation Report No. S11-13 Compiled & Edited by B. Noake (Conservation Officer – Threatened Species), A. Rosenthal (Conservation Officer – Threatened Species), M. Parsons (Head of Moth Conservation) & Dr. N. Bourn (Director of Conservation) March 2011 Butterfly Conservation Company limited by guarantee, registered in England (2206468) Registered Office: Manor Yard, East Lulworth, Wareham, Dorset. BH20 5QP Charity registered in England and Wales (254937) and in Scotland (SCO39268) www.butterfly-conservation.org Noake. B., Rosenthal, A., Parsons, M. & Bourn, N. (eds.) (2011) Lepidoptera Conservation Bulletin Number 11: April 2010 – March 2011, Butterfly Conservation, Wareham. (Butterfly Conservation Report No. S11-13) Contents 1 INTRODUCTION ................................................................................................................................................. 1 2 ACKNOWLEDGMENTS ....................................................................................................................................... 2 3 CONSERVATION ACTION FOR UK BIODIVERSITY ACTION PLAN LEPIDOPTERA ................................................... 2 3.1 UPDATE ON UK BAP MOTHS – A SUMMARY FOR THE YEAR 2010 ........................................................................................... 3 Anania funebris.........................................................................................................................................................