Microbial Diversity of Paulownia Spp. Leaves – a New Source of Green Manure
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

S41598-019-53283-2.Pdf
www.nature.com/scientificreports OPEN Time-coursed transcriptome analysis identifes key expressional regulation in growth cessation and dormancy induced by short days in Paulownia Jiayuan Wang1, Hongyan Wang2, Tao Deng3, Zhen Liu1* & Xuewen Wang 3,4* Maintaining the viability of the apical shoot is critical for continued vertical growth in plants. Terminal shoot of tree species Paulownia cannot regrow in subsequent years. The short day (SD) treatment leads to apical growth cessation and dormancy. To understand the molecular basis of this, we further conducted global RNA-Seq based transcriptomic analysis in apical shoots to check regulation of gene expression. We obtained ~219 million paired-end 125-bp Illumina reads from fve time-courses and de novo assembled them to yield 49,054 unigenes. Compared with the untreated control, we identifed 1540 diferentially expressed genes (DEGs) which were found to involve in 116 metabolic pathways. Expression of 87% of DEGs exhibited switch-on or switch-of pattern, indicating key roles in growth cessation. Most DEGs were enriched in the biological process of gene ontology categories and at later treatment stages. The pathways of auxin and circadian network were most afected and the expression of associated DEGs was characterised. During SD induction, auxin genes IAA, ARF and SAURs were down-regulated and circadian genes including PIF3 and PRR5 were up-regulated. PEPC in photosynthesis was constitutively upregulated, suggesting a still high CO2 concentrating activity; however, the converting CO2 to G3P in the Calvin cycle is low, supported by reduced expression of GAPDH encoding the catalysing enzyme for this step. This indicates a de-coupling point in the carbon fxation. -

An Investigation Into the Suitability of Paulownia As an Agroforestry Species for UK & NW European Farming Systems
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/311558333 An investigation into the suitability of Paulownia as an agroforestry species for UK & NW European farming systems Thesis · May 2016 DOI: 10.13140/RG.2.2.31955.78882 CITATION READS 1 2,475 1 author: Janus Bojesen Jensen Coventry University 1 PUBLICATION 1 CITATION SEE PROFILE Some of the authors of this publication are also working on these related projects: An Exploration of the Potential of Quantum-Based Agriculture for Sustainable Global Food Production View project Quantum Agriculture View project All content following this page was uploaded by Janus Bojesen Jensen on 10 December 2016. The user has requested enhancement of the downloaded file. An investigation into the suitability of Paulownia as an agroforestry species for UK & NW European farming systems Janus Bojesen Jensen, B.B.A. (Beirut) Submitted to the Department of Agriculture & Business Management, SRUC, in partial fulfilment of the requirements for the degree of Master of Science SRUC, 2016 Acknowledgements I would like to thank Dr. Jo Smith for her invaluable support and guidance throughout this project. I would also like to express my heartfelt gratitude to Dr. Lou Ralph and all the teaching staff at SRUC for my experience and learning as a student at SRUC over the last three years. Lastly, I would like to express my thanks and appreciation to all the participants who were involved in this study and shared their time and knowledge with a particular acknowledgement to Dr. Ian Lane for all his contributions and for going the ‘extra country mile’ with me on two occasions. -

The Effects of Wildland Fire and Other Disturbances on the Nonnative Tree Paulownia Tomentosa and Impacts on Native Vegetation
The Effects of Wildland Fire and Other Disturbances on the Nonnative Tree Paulownia tomentosa and Impacts on Native Vegetation THESIS Presented in Partial Fulfillment of the Requirements for the Degree Master of Science in the Graduate School of The Ohio State University By Angela R. Chongpinitchai Graduate Program in Environment and Natural Resources The Ohio State University 2012 Master's Examination Committee: Roger Williams, Advisor Joanne Rebbeck David Hix Stephen Matthews Copyrighted by Angela Rose Chongpinitchai 2012 Abstract Increases in nonnative plant species to ecosystems of the United States have become a concern for the management of natural vegetation and ecological processes. Many exotic plant species are invasive, rapidly colonizing an area and spreading beyond the point of introduction. Nonindigenous plants can change the dynamics of soil properties, alter fire regimes, and interrupt ecological processes, such as nutrient and hydrologic cycles. These exotic plants often have a competitive edge over native species, due to adaptations in their native geographical range or because of a lack of natural predators in introduced habitats. Changes to microclimate conditions and direct competition from exotic plants often lead to a decrease in species richness in a habitat. Although this has been an issue for decades, little is known about many exotic plant species, particularly how they interact with natural vegetation in introduced habitats. Increased anthropogenic activity, such as logging, road construction, and prescribed burns, tends to facilitate invasions of exotic species by creating disturbed areas and reducing biodiversity. Paulownia tomentosa, a tree native to China, was first introduced to the U.S. in the 1840s as an ornamental; in recent decades the species has expanded its range since escaping cultivation. -

Kadoorie Farm and Botanic Garden, 2004. Report of Rapid Biodiversity Assessments at Dachouding and Sanyue Nature Reserves, Northwest Guangdong, China, April 2001
Report of Rapid Biodiversity Assessments at Dachouding and Sanyue Nature Reserves, Northwest Guangdong, China, April 2001 Kadoorie Farm and Botanic Garden in collaboration with Zhongshan University Zhaoqing Forestry Bureau February 2004 South China Forest Biodiversity Survey Report Series: No. 37 (Online Simplified Version) Report of Rapid Biodiversity Assessments at Dachouding and Sanyue Nature Reserves, Northwest Guangdong, China, April 2001 Editors Bosco P.L. Chan, Ng Sai-Chit, Michael W.N. Lau and John R. Fellowes Contributors Kadoorie Farm and Botanic Garden: Michael W.N. Lau (ML) Bosco P.L. Chan (BC) John R. Fellowes (JRF) Lee Kwok Shing (LKS) Ng Sai-Chit (NSC) Roger Kendrick (RCK) Zhongshan University: Chang Hong (CH) Voluntary specialists: Graham T. Reels (GTR) Keith D.P. Wilson (KW) Background The present report details the findings of a trip to Northwest Guangdong by members of Kadoorie Farm and Botanic Garden (KFBG) in Hong Kong and their colleagues, as part of KFBG's South China Biodiversity Conservation Programme (renamed the China Programme in 2003). The overall aim of the programme is to minimise the loss of forest biodiversity in the region, and the emphasis in the first three years is on gathering up-to-date information on the distribution and status of fauna and flora. Citation Kadoorie Farm and Botanic Garden, 2004. Report of Rapid Biodiversity Assessments at Dachouding and Sanyue Nature Reserves, Northwest Guangdong, China, April 2001 . South China Forest Biodiversity Survey Report Series (Online Simplified Version): No. 37. KFBG, Hong Kong SAR, ii + 33 pp. Copyright Kadoorie Farm and Botanic Garden Corporation Lam Kam Road, Tai Po, N.T., Hong Kong February 2004 - i - Contents Objectives ……………………………………………………………………………………. -

Princess-Tree (Paulownia Tomentosa)
New Plant Invaders to Watch For Princess-tree (Paulownia tomentosa) The Threat: Princess-tree is a small to medium sized deciduous tree that can grow 30 to 60 feet tall. It has been planted as an ornamental but has been found to escape and colonize natural areas. Seeds are spread by wind and water. It’s ability to sprout from buds on stems and roots enhance its ability to survive, spread, and out-compete native plant species. Massachusetts is near the northern limit for winter hardiness of princess-tree and projected climate warming may allow this species to proliferate here. Large (up to 12 or more inches long) heart-shaped, broadly oval, or slightly lobed leaves are arranged in pairs along the stem. The underside of the leaves are hairy. Flowers are pale violet or whitish, tubular with five petals, and open in the Photo: James H. Miller, USDA Forest Service, spring. Bugwood.org What to do : Learn to recognize the distinguishing features of princess-tree shown in the images on this page. If found, immediately report the finding to Lou Wagner, Mass Audubon Regional Scientist, at 978-927-1122 Extension Photo: Leslie J. Mehrhoff, Univer- 2705, or [email protected]. Fruits turn brown and persist through Fruits occur in 1 to 2 inch long winter, opening in late winter to clusters. The fruits distinguish release many small winged seeds. princess tree from Catalpa, which has similar leaves but long, slender, bean-like fruits. Photo: James H. Miller, USDA Forest Service, Bugwood.org Photo: James H. Miller, USDA Forest Service, Bugwood.org Twigs are glossy gray-brown with numerous white dots (lenticels). -

Ecology and Invasive Potential of Paulownia Tomentosa
ECOLOGY AND INVASIVE POTENTIAL OF PAULOWNIA TOMENTOSA (SCROPHULARIACEAE) IN A HARDWOOD FOREST LANDSCAPE A dissertation presented to the faculty of the College of Arts and Sciences of Ohio University In partial fulfillment of the requirements for the degree Doctor of Philosophy A. Christina W. Longbrake August 2001 © 2001 A. Christina W. Longbrake All Rights Reserved ECOLOGY AND INVASIVE POTENTIAL OF PAULOWNIA TOMENTOSA (SCROPHULARIACEAE) IN A HARDWOOD FOREST LANDSCAPE BY A. CHRISTINA W. LONGBRAKE This dissertation has been approved for the Department of Environmental and Plant Biology and the College of Arts and Sciences by Brian C. McCarthy Associate Professor of Environmental and Plant Biology Leslie A. Flemming Dean, College of Arts and Sciences LONGBRAKE, A. CHRISTINA W. Ph.D. August 2001. Environmental and Plant Biology Ecology and invasive potential of Paulownia tomentosa (Scrulariaceae) in a hardwood forest landscape. (174 pp.) Director of Dissertation: Brian C. McCarthy Introduction of non-native species is the oldest form of human-induced global change. From the exchange of agricultural crops and domestic animals, to the accidental introduction of weeds and microbes, non-native species have been incorporated into the floras and faunas of all continents and most oceanic islands. These organisms can have marked effects on ecosystems. I wanted to address the following facets of non-native species invasion: (1) What characteristics of ecosystems make them more susceptible to non-native species invasion? and (2) What characteristics of the invader allow invasion? To address these questions, I used a gradient of anthropogenic disturbance common in southeastern Ohio forests: intact secondary forest, forest edge, and aggrading clear cuts. -

Paulownia Control Plan
Scientific Name: Paulownia tomentosa Common Name: Princess Tree Updated: 12/30/2015 A. Priority: B B. Description – Native to eastern Asia, Paulownia tomentosa has been widely planted for horticultural purposes in North America from Montreal to Florida and west to Missouri and Texas. This tree is moderately cold-hardy so it has spread principally in the Eastern and Southern portions of the United States. In North Carolina it poses a particular problem in the foothill and mountain regions. Paulownia tomentosa is capable of flowering within 8 to 10 years and a mature tree can produce millions of seeds. The seeds are small and winged, dispersing easily in the wind. This aggressive tree grows rapidly (up to 15 feet per year) in all types of disturbed habitats. Princess tree may reach a height of up to 50 feet. The bark of this tree is characteristically gray with shallow, shiny ribs. The leaves are large (5-10 inches long on mature trees), heart-shaped, and oppositely arranged along the branches. The edges of the leaves often have blunt “horns” on each side. Stump sprouts and young plants have extremely large leaves that can be up to 32 inches long. This tree flowers in April and May, usually before its leaves have fully emerged. The very, large, light purple flowers are distinctively sticky and hairy on the outside. These flowers are arranged in pyramidal clusters that are about 10 to 15 inches long. The fruits appear from April through June and the hulls persist in large brown clusters through the winter and into early spring. -

The History of the Empress Tree (Paulownia) in the USA
Forests, Trees and Livelihoods ISSN: 1472-8028 (Print) 2164-3075 (Online) Journal homepage: http://www.tandfonline.com/loi/tftl20 Ornamental, crop, or invasive? The history of the Empress tree (Paulownia) in the USA Whitney Adrienne Snow To cite this article: Whitney Adrienne Snow (2015) Ornamental, crop, or invasive? The history of the Empress tree (Paulownia) in the USA, Forests, Trees and Livelihoods, 24:2, 85-96, DOI: 10.1080/14728028.2014.952353 To link to this article: http://dx.doi.org/10.1080/14728028.2014.952353 Published online: 08 Sep 2014. Submit your article to this journal Article views: 64 View related articles View Crossmark data Full Terms & Conditions of access and use can be found at http://www.tandfonline.com/action/journalInformation?journalCode=tftl20 Download by: [University of Nebraska, Lincoln] Date: 10 October 2015, At: 11:22 Forests, Trees and Livelihoods, 2015 Vol. 24, No. 2, 85–96, http://dx.doi.org/10.1080/14728028.2014.952353 Ornamental, crop, or invasive? The history of the Empress tree (Paulownia) in the USA Whitney Adrienne Snow* Department of History, Midwestern State University, Wichita Falls, TX, USA The Paulownia tree first arrived in the USA in the early nineteenth century and quickly transitioned from an exotic oddity to a beloved ornamental by some and a reviled invasive species by others. Many gardeners and horticulturalists praised the tree for its rapid growth and beautiful lavender blossoms. Critics thought the non-native tree a threat to native flora and sought its eradication. One species, Paulownia tomentosa,is invasive and has been especially vilified by organizations ranging from the National Park Service and National Forests Service to the US Department of Agriculture. -

Paulownia Tomentosa
Paulownia tomentosa INTRODUCTORY DISTRIBUTION AND OCCURRENCE BOTANICAL AND ECOLOGICAL CHARACTERISTICS FIRE EFFECTS AND MANAGEMENT MANAGEMENT CONSIDERATIONS APPENDIX: FIRE REGIME TABLE REFERENCES INTRODUCTORY AUTHORSHIP AND CITATION FEIS ABBREVIATION NRCS PLANT CODE COMMON NAMES TAXONOMY SYNONYMS LIFE FORM FEDERAL LEGAL STATUS OTHER STATUS Princesstree in postfire habitat in Linville Gorge Wilderness Area, North Carolina. Photo by Dane Kuppinger. AUTHORSHIP AND CITATION: Innes, Robin J. 2009. Paulownia tomentosa. In: Fire Effects Information System, [Online]. U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory (Producer). Available: http://www.fs.fed.us/database/feis/ [ 2010, February 8]. FEIS ABBREVIATION: PAUTOM NRCS PLANT CODE [140]: PATO2 COMMON NAMES: princesstree princess tree princess-tree paulownia royal paulownia empress tree imperial-tree kiri tree TAXONOMY: The scientific name of princesstree is Paulownia tomentosa (Thunb.) Sieb. & Zucc. ex Steud. [45,73]. A review [3] stated that botanists have historically debated the taxonomic classification of princesstree, placing it within either the figwort (Scrophulariaceae) or trumpet-creeper (Bignoniaceae) family. Based on floral anatomy, embryo morphology, and seed morphology, princesstree is placed in Scrophulariaceae, a family of mostly herbaceous species [3,62,158]. Princesstree is a popular ornamental, and several cultivars have been developed [62,115,123]. SYNONYMS: Paulownia imperialis Sieb. & Zucc. [62] LIFE FORM: Tree FEDERAL LEGAL STATUS: None OTHER STATUS: Information on state-level noxious weed status of plants in the United States is available at Plants Database. DISTRIBUTION AND OCCURRENCE SPECIES: Paulownia tomentosa GENERAL DISTRIBUTION HABITAT TYPES AND PLANT COMMUNITIES GENERAL DISTRIBUTION: Princesstree is nonnative in North America. It occurs from Montreal, Canada, south to Florida and west to Texas and Indiana; it has also been planted in coastal Washington [101] and California [62]. -
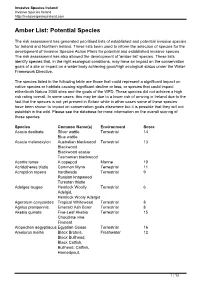
Amber List: Potential Species
Invasive Species Ireland Invasive Species Ireland http://invasivespeciesireland.com Amber List: Potential Species The risk assessment has generated prioritised lists of established and potential invasive species for Ireland and Northern Ireland. These lists been used to inform the selection of species for the development of Invasive Species Action Plans for potential and established invasive species. The risk assessment has also allowed the development of 'amber list' species. These lists identify species that, in the right ecological conditions, may have an impact on the conservation goals of a site or impact on a water body achieving good/high ecological status under the Water Framework Directive. The species listed in the following table are those that could represent a significant impact on native species or habitats causing significant decline or loss; or species that could impact either/both Natura 2000 sites and the goals of the WFD. These species did not achieve a high risk rating overall. In some cases, this may be due to a lower risk of arriving in Ireland due to the fact that the species is not yet present in Britain while in other cases some of these species have been shown to impact on conservation goals elsewhere but it is possible that they will not establish in the wild. Please see the database for more information on the overall scoring of these species. Species Common Name(s) Environment Score Acacia dealbata Silver wattle Terrestrial 14 Blue wattle Acacia melanoxylon Australian blackwood Terrestrial 13 Blackwood Blackwood -

Paulownia As a Medicinal Tree: Traditional Uses and Current Advances
European Journal of Medicinal Plants 14(1): 1-15, 2016, Article no.EJMP.25170 ISSN: 2231-0894, NLM ID: 101583475 SCIENCEDOMAIN international www.sciencedomain.org Paulownia as a Medicinal Tree: Traditional Uses and Current Advances Ting He 1, Brajesh N. Vaidya 1, Zachary D. Perry 1, Prahlad Parajuli 2 1* and Nirmal Joshee 1College of Agriculture, Family Sciences and Technology, Fort Valley State University, Fort Valley, GA 31030, USA. 2Department of Neurosurgery, Wayne State University, 550 E. Canfield, Lande Bldg. #460, Detroit, MI 48201, USA. Authors’ contributions This work was carried out in collaboration between all authors. All authors read and approved the final manuscript. Article Information DOI: 10.9734/EJMP/2016/25170 Editor(s): (1) Marcello Iriti, Professor of Plant Biology and Pathology, Department of Agricultural and Environmental Sciences, Milan State University, Italy. Reviewers: (1) Anonymous, National Yang-Ming University, Taiwan. (2) P. B. Ramesh Babu, Bharath University, Chennai, India. Complete Peer review History: http://sciencedomain.org/review-history/14066 Received 20 th February 2016 Accepted 31 st March 2016 Mini-review Article Published 7th April 2016 ABSTRACT Paulownia is one of the most useful and sought after trees, in China and elsewhere, due to its multipurpose status. Though not regarded as a regular medicinal plant species, various plant parts (leaves, flowers, fruits, wood, bark, roots and seeds) of Paulownia have been used for treating a variety of ailments and diseases. Each of these parts has been shown to contain one or more bioactive components, such as ursolic acid and matteucinol in the leaves; paulownin and d- sesamin in the wood/xylem; syringin and catalpinoside in the bark. -

Paulownia Tomentosa, an Exotic Species in North America, Is Spreading in fi Re-Opened Landscapes of Southern Appalachia
Paulownia tomentosa, an exotic species in North America, is spreading in fi re-opened landscapes of southern Appalachia. Nature in a Name: Paulownia tomentosa—Exotic Tree, Native Problem Summary While awareness of fi re’s importance in dry Appalachian forests, and the application of fi re as a restoration tool have increased over the last two decades, so too has the post-fi re invasion of Paulownia tomentosa (Princess tree). For the last ten years, managers have witnessed Paulownia invasion grow following fi re events. To understand this better, the team studied fi ve life history transitions for the species: seed dispersal, seed germination, seed survival over time through incorporation in the seed bank, initial habitat requirements, and seedling persistence to maturity. Paulownia seeds were found to disperse over two miles from their source tree. Fires of greater severity promote conditions Paulownia favors—exposed soil free of organic litter, and openings in the canopy cover that allow ample light. Subsequent persistence by Paulownia is greatest in the drier and more exposed areas, such as ridges and steep slopes. Fire Science Brief Issue 116 July 2010 Page 1 www.fi rescience.gov Key Findings • Paulownia seeds were widely dispersed. Though the majority of these wind-dispersed seeds did not travel far from the parent tree, seedlings were frequently encountered two miles from the nearest mature (seed producing) tree. • Paulownia trees had the greatest presence and numbers on high benches, crests and high slopes—places that are drier and experience greater fi re intensity. • Canopy cover was the most signifi cant predictor of Paulownia invasion overall.