Jenner Vaccine Foundation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Old Road Campus
Old Road Campus 4a, 4b, 4c, U5 n o t Oxford City Centre g OLD ROAD n i d 4a, 4b, 4c, U5 a e H K O L L A D R O W AD E M I L 4,4a,4b,4c, U1X,U5 A41 42 4,4a,4b,4c,U5 Rin D 4 g R oad 6 7 1 13 3 11 C H U R C H I L L 900 D 2 R I 700, 900 V E E O x f o 5 A C rd 12 C i ty 10 C e n t re B 8 CAR PARK C 9 h u r c R F h i O l l O S H E o V s N E p L i T t DRI a VE ENTRANCE ROOSEVELT DRIVE l 900, ST2 Index 1 The Triangle Nursery 9 Old Road Campus Estates Annexe 13 Boundary Brook House Interserve Joint Research Office Kennedy Institute 2 - Research Services, Medical Sciences Division Old Road Campus Research Building 10 - Clinical Trials and Research Governance 3 New Richards Building Department of Oncology - Human Tissue Governance CRUK/MRC Oxford Institute for Radiation Oncology - Medical Sciences Division Business Development 4 NDM Research Building Institute of Biomedical Engineering Nuffield Department of Primary Care Health Sciences Target Discovery Institute Jenner Institute Medical Sciences Divisional Safety Officers Centre for Tropical Medicine and Global Health Bodleian Knowledge Centre (Library Services) Medical Sciences Division IT Services 5 Wellcome Centre for Human Genetics (WHG) Ludwig Institute for Cancer Research Structural Genomics Consortium 6 Henry Wellcome Building for Molecular Physiology Nuffield Department of Surgical Sciences Loading Bays and Delivery Offices of the Nuffield Professor of Medicine ENTRANCE VIA BUILDING 5 11 Big Data Institute A Wellcome Trust Centre for Human Genetics 7 Henry Wellcome Building for Particle Imaging -

The 6-In-1 Vaccine Study
Oxford Vaccine Group University of Oxford Centre for Clinical Vaccinology and Tropical Medicine, Churchill Hospital, Headington, Oxford OX3 7LE Telephone: 01865 611400 [email protected] www.ovg.ox.ac.uk The 6-in-1 vaccine study Study Information Booklet We are inviting infants aged 8-13 weeks of age to take part in a study comparing two licensed combination vaccines that can be given in the routine UK immunisation schedule. These vaccines protect against 6 different infections in the one injection. Before you decide to take part in this study, it is important for you to understand what the study is about and what participation would involve. Please take time to read the information carefully, and discuss with others if you wish. If you have any questions please contact the study team. Thank you for taking the time to consider this study. Contact the local study team at: Oxford Vaccine Group CCVTM, Churchill Hospital, Oxford, OX3 7LE 01865 611400 [email protected] www.ovg.ox.ac.uk/recruiting-studies Page 1 of 11 The 6-in-1 vaccine study; OVG 2018/05; Participant Information Booklet; REC Ref: 19/SC/0052; IRAS ID: 252324; Version 2.0 dated 23-APR-2019 Oxford Vaccine Group University of Oxford Centre for Clinical Vaccinology and Tropical Medicine, Churchill Hospital, Headington, Oxford OX3 7LE Telephone: 01865 611400 [email protected] www.ovg.ox.ac.uk Summary • This study will help us to better understand the interaction between ‘6- in-1’ vaccines and the Meningococcal B vaccine in the routine UK immunisation schedule • We will be enrolling 240 healthy babies aged 8 – 13 weeks into this study • Babies will receive all their infant immunisations in their own home or in a convenient location until 12 months of age • There will be six study visits including 4 vaccine visits and 2 visits for blood sampling (we will use a topical anaesthetic cream to numb the skin for blood sampling) Why has my child been invited to take part? You have been approached as your child is in the age range for this study and lives in the Thames Valley. -
Astrazeneca-Oxford Vaccine Approved for Use in the U.K
P2JW366000-6-A00100-17FFFF5178F ****** THURSDAY,DECEMBER 31,2020~VOL. CCLXXVI NO.154 WSJ.com HHHH $4.00 DJIA 30409.56 À 73.89 0.2% NASDAQ 12870.00 À 0.2% STOXX 600 400.25 g 0.3% 10-YR. TREAS. À 3/32 , yield 0.926% OIL $48.40 À $0.40 GOLD $1,891.00 À $10.50 EURO $1.2300 YEN 103.21 Deadly Attack at Airport Targets New Yemen Government U.S. IPO What’s News Market Reaches Business&Finance Record nvestorspiled into IPOs Iat a record rate in 2020, with companies raising Total $167.2 billion via 454 of- ferings on U.S. exchanges this year through Dec. 24. Few see signs of letup Few expect the euphoria after companies raise to wear off soon. A1 more than $167 billion Detenteisending in the global fight over tech taxes, despite pandemic with Franceresuming collec- tion of itsdigital-services tax BY MAUREEN FARRELL and the U.S. poised to retali- atewith tariffs.Other coun- Defying expectations,inves- tries areset to join the fray. A1 S tors piled intoinitial public of- China finished 2020 PRES feringsatarecordrateiN with a 10th consecutive TED 2020, and few expect the eu- month of expansion in its CIA phoria to wear off soon. manufacturing sector. A7 SO Companies raised $167.2 AS TheEUand China agreed TENSIONS HIGH: People fled after an explosion Wednesday at the airport in Aden, Yemen, moments after members of the billion through 454 offerings in principle on an invest- country’s newly sworn-in cabinet arrived. At least 22 people were killed, but all the members of the cabinet were safe. -

Annual Meeting
Volume 97 | Number 5 Volume VOLUME 97 NOVEMBER 2017 NUMBER 5 SUPPLEMENT SIXTY-SIXTH ANNUAL MEETING November 5–9, 2017 The Baltimore Convention Center | Baltimore, Maryland USA The American Journal of Tropical Medicine and Hygiene The American Journal of Tropical astmh.org ajtmh.org #TropMed17 Supplement to The American Journal of Tropical Medicine and Hygiene ASTMH FP Cover 17.indd 1-3 10/11/17 1:48 PM Welcome to TropMed17, our yearly assembly for stimulating research, clinical advances, special lectures, guests and bonus events. Our keynote speaker this year is Dr. Paul Farmer, Co-founder and Chief Strategist of Partners In Health (PIH). In addition, Dr. Anthony Fauci, Director of the National Institute of Allergy and Infectious Diseases, will deliver a plenary session Thursday, November 9. Other highlighted speakers include Dr. Scott O’Neill, who will deliver the Fred L. Soper Lecture; Dr. Claudio F. Lanata, the Vincenzo Marcolongo Memorial Lecture; and Dr. Jane Cardosa, the Commemorative Fund Lecture. We are pleased to announce that this year’s offerings extend beyond communicating top-rated science to direct service to the global community and a number of novel events: • Get a Shot. Give a Shot.® Through Walgreens’ Get a Shot. Give a Shot.® campaign, you can not only receive your free flu shot, but also provide a lifesaving vaccine to a child in need via the UN Foundation’s Shot@Life campaign. • Under the Net. Walk in the shoes of a young girl living in a refugee camp through the virtual reality experience presented by UN Foundation’s Nothing But Nets campaign. -

Jenner Institute Complementary Vaccines Platform Technologies
WHO R&D Blueprint: Janssen Vaccines – Jenner Institute complementary Vaccines Platform Technologies Janssen Vaccines: Jenner Institute: Olga Popova Prof. Sarah Gilbert Jerome Custers WHO Geneva, 21 July 2016 Background • Jenner Institute & Janssen Vaccines presented respective proposals to WHO R&D Blueprint Workshop in April 2016, and were invited to join forces for Round 2 submission • Example of alignment, coordination and partnership between public and private sector stakeholders • Understanding nature of vaccine development, established complementary end‐to‐end skills and capabilities • Long‐term, sustainable & consistent approach and funding • High‐level flexible proposal with illustrative examples • «Bona fide»: collaborative framework to be developed JOINTLY TOWARDS TANGIBLE OUTCOMES x GLOBAL PUBLIC HEALTH 2 Success factors • Available platforms and previous experience with pathogens • Ability to invest time and resources, leverage expertise, minimise opportunity costs and ensure business continuity • Appropriate and functionable operational model, speed • Lean governance, partner alignment and milestone orientation INTERNAL • Reliable & qualified partners, durable commitments • Long‐term reliable funding (min 5‐year horizon) • Resolving vaccination indemnification / liability issue • Consistency in pathogen prioritisation and defined, consistent pre‐ established endpoint commitment • Clear and accelerated / streamlined regulatory pathways, conditions & predictability of licensure EXTERNAL • Anticipated deployment plans and community -
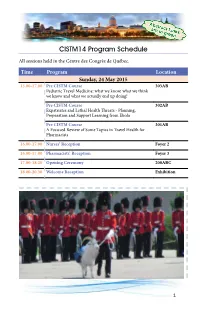
CISTM14 Program Schedule
CISTM14 Program Schedule All sessions held in the Centre des Congrès de Québec. Time Program Location Sunday, 24 May 2015 13.00-17.00 Pre CISTM Course 303AB Pediatric Travel Medicine: what we know, what we think we know and what we actually end up doing! Pre CISTM Course 302AB Expatriates and Lethal Health Threats - Planning, Preparation and Support Learning from Ebola Pre CISTM Course 301AB A Focused Review of Some Topics in Travel Health for Pharmacists 16.00-17.00 Nurses’ Reception Foyer 2 16.00-17.00 Pharmacists’ Reception Foyer 3 17.00-18.20 Opening Ceremony 200ABC 18.00-20.30 Welcome Reception Exhibition 1 Time Program Location Monday, 25 May 2015 MTH1 Meet The History 301AB 8.00-8.45 History of the Quarantine Station at Grosse Ile Marc Desmeules, Canada 8.00-8.45 CDC Yellow Book 302AB Gary Brunette, United States of America COD1 Case of the Day 303AB 8.00-8.45 Pre-Travel Rogelio Lopez-Velez, Spain PL1 Plenary 200ABC 9.00-10.30 Our Shrinking World: Health in the 21st Century Chairs: David R. Shlim, United States of America Leo Visser, The Netherlands PL1.01 Measuring global health: The global is local [Change your mindset! It’s time for a reality check] Louis Loutan, Switzerland • Outline the most important trends and health challenges in the world using gapminder/health metrics • Appraise how these changes (global health transitions, global population growth, urbanization, changes in animal helath and human-animal population interactions, and climate change) will influence global migration and travel medicine now and in the future • Integrate global health concepts into thinking about travel medicine 2 Time Program Location Monday, 25 May 2015, continued Cont. -

Sir Bryn Terfel Premieres John Rutter's 'Joseph's Carol', Dedicated to the Oxford Vaccine Team in Celebratory Concert Fr
Sir Bryn Terfel premieres John Rutter’s ‘Joseph’s Carol’, dedicated to the Oxford vaccine team in celebratory concert from the Oxford Philharmonic Orchestra Friday 18 December 2020, 18:30 Streamed on Oxford Philharmonic Orchestra’s YouTube Channel: bit.ly/OPOVaccineTribute Elgar Chanson de Matin William Henry Monk Abide with Me Rodgers & Hammerstein You’ll Never Walk Alone John Rutter Joseph’s Carol WORLD PREMIERE John Rutter Look to the Day Handel Hallelujah Chorus Sir Bryn Terfel bass-baritone Oxford Philharmonic Orchestra Maxim Vengerov violin Choir of Merton College, Oxford John Rutter conductor Alexandra Lowe soprano Marios Papadopoulos conductor Alexander Olleson treble John Suchet presenter In recognition of the formidable work accomplished by the team of scientists at the University of Oxford on their Covid-19 vaccine, the Oxford Philharmonic Orchestra will stream a celebratory concert on Friday 18 December, recorded in the city’s historic Sheldonian Theatre. Performed by bass-baritone Sir Bryn Terfel, the short concert features the premiere of John Rutter’s Joseph’s Carol, written in tribute to the Oxford Vaccine Group, the Jenner Institute and the RECOVERY team. The words by John Rutter recount the long and weary journey of Joseph and Mary to Bethlehem before the birth of the baby Jesus, echoing the programme’s journey from struggle through to hope. Bryn Terfel also joins the Orchestra and the Choir of Merton College, Oxford, in a rousing programme from Rodgers & Hammerstein’s You’ll Never Walk Alone (with Jette Parker Young Artist Alexandra Lowe) to Handel’s Hallelujah Chorus. Sir Bryn and the Orchestra are also joined in the hymn of comfort, Abide with Me, by chorister Alexander Olleson of Christ Church Cathedral Choir, the recent winner of BBC Young Chorister Of The Year 2020. -

Immunomodulatory Effects of the Hepatitis C Virus (HCV) Core Protein
Immunomodulatory Effects of the Hepatitis C Virus (HCV) Core Protein By Rachel Elizabeth Owen A thesis submitted for the degree of Doctor of Philosophy at the University of London September 2003 The Edward Jenner Institute for Vaccine Research University College London ProQuest Number: U642330 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. uest. ProQuest U642330 Published by ProQuest LLC(2015). Copyright of the Dissertation is held by the Author. All rights reserved. This work is protected against unauthorized copying under Title 17, United States Code. Microform Edition © ProQuest LLC. ProQuest LLC 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 48106-1346 Abstract Hepatitis C virus establishes a persistent infection in approximately 80% of infected individuals. It is unclear why the immune system is frequently unable to mediate a response capable of controlling HCV replication. Dendritic cells (DCs) may be an extrahepatic site of viral replication and it is hypothesised that expression of the core protein within DCs may impair their functions resulting in inadequate immune responses. To investigate potential immunomodulatory effects of the core protein on DCs, replication-deficient adenoviral vectors co-expressing different versions of the core protein (full-length, truncated and a version lacking residues 125-144) with GFP were constructed. Whilst these adenoviruses were being generated, experiments were carried out with an adenoviral vector expressing all the HCV structural proteins (Ad- CE1E2). -

TB Vaccine Development
Impact Objectives • Work towards developing an effective vaccine against tuberculosis (TB) • Confirm the safety and immunogenicity of delivering the MVA85A vaccine by aerosol direct to the respiratory mucosa • Ultimately produce an effective, safe and accessible vaccine for the global prevention of TB Overcoming the challenges of tuberculosis (TB) vaccine development Clinician and Professor of Vaccinology at the University of Oxford in the UK, Helen McShane is using her 26 years of expertise to lead a research team in their quest to develop, assess and apply a new TB vaccine Could you give Could you expand on your own previous significantly less immunogenic in South a brief overview work into the effectiveness of the BCG African infants than it had been in UK adults. of TB in terms vaccination and the work you have led of prevalence developing a booster vaccine? Despite these results, we have learnt a huge of the disease amount from the conduct of this trial, and today, current In 2002, we started a clinical trial programme nine additional papers have been published treatments and with MVA85A, the first subunit vaccine from the data arising from this trial. We their effectiveness, and any drawbacks? candidate TB vaccine to be tested in clinical have gained important insights into immune trials. This vaccine was designed to boost the correlates of risk of TB disease from samples Tuberculosis kills more people today than any effects of BCG. After a careful programme of taken during this trial, how to diagnose other infectious pathogen. The emergence trials in the UK, including testing this vaccine TB in infants, and the role of Quantiferon of multi and extensively drug-resistant in M tb latently infected people, we then testing in infants exposed to TB. -

Hepcidin Deficiency and Iron Deficiency Do Not Alter Tuberculosis Susceptibility in a Murine M
RESEARCH ARTICLE Hepcidin deficiency and iron deficiency do not alter tuberculosis susceptibility in a murine M. tb infection model Rachel Harrington-Kandt1, Elena Stylianou1, Lucy A. Eddowes2, Pei Jin Lim2, Lisa Stockdale3, Nawamin Pinpathomrat1, Naomi Bull1, Janet Pasricha1, Marta Ulaszewska1, Yulia Beglov4, Sophie Vaulont5, Hal Drakesmith2☯*, Helen McShane1☯* a1111111111 1 Jenner Institute, University of Oxford, Oxford, United Kingdom, 2 MRC Human Immunology Unit, Weatherall Institute of Molecular Medicine, University of Oxford, Oxford, United Kingdom, 3 Department of a1111111111 Immunology and Infection, London School of Hygiene & Tropical Medicine, London, United Kingdom, a1111111111 4 Wellcome Trust Centre for Human Genetics, University of Oxford, Oxford, United Kingdom, 5 Institut a1111111111 Cochin, INSERM 567, CNRS 8104, Universite Paris 5, Paris, France a1111111111 ☯ These authors contributed equally to this work. * [email protected] (HD); [email protected] (HMcS) OPEN ACCESS Abstract Citation: Harrington-Kandt R, Stylianou E, Eddowes LA, Lim PJ, Stockdale L, Pinpathomrat N, Tuberculosis (TB), caused by the macrophage-tropic pathogen Mycobacterium tuberculosis et al. (2018) Hepcidin deficiency and iron (M.tb) is a highly prevalent infectious disease. Since an immune correlate of protection deficiency do not alter tuberculosis susceptibility in or effective vaccine have yet to be found, continued research into host-pathogen interac- a murine M.tb infection model. PLoS ONE 13(1): tions is important. Previous literature reports links between host iron status and disease e0191038. https://doi.org/10.1371/journal. pone.0191038 outcome for many infections, including TB. For some extracellular bacteria, the iron regula- tory hormone hepcidin is essential for protection against infection. -

Download a PDF of Our Community Brochure
Engagement with the communities of Oxford and Oxfordshire Did you know? St Giles’ Fair began as the parish feast of St Giles, first recorded in 1624. From the 1780s it became a toy fair, with general amusements for children. In the next century its focus shifted towards adults, with entertainment, rides and stalls. In the late 1800s there were calls for the fair to be stopped on the grounds that it encouraged rowdy behaviour. During Victorian times engineering advances brought the forerunners of today’s rides. Today the huge pieces of machinery fill St Giles’ with sparkling lights for a few days each year, and whizz within feet of ancient college buildings. The stone heads around the Sheldonian Theatre now number thirteen (there were originally fourteen, but one was removed to make way for the adjoining Clarendon Building.) It is not known what they were intended to represent – they might be gods, wise men, emperors or just boundary markers. The original heads were made by William Byrd and put up in 1669. Did you Replacements put up in 1868 were made in poor stone, know? which crumbled away; in 1972 the current set, carved by Michael Black of Oxford, were erected. More on page 4 STARGAZING AND SPIN-OUTS PAGE 1 Contents 2 Introduction from the Vice-Chancellor 3 Foreword from the Chair of the Community Engagement Group 5 Part 1: Part of the fabric of the city Part of the fabric 6 800 years of history of the 8 Economic impact city 9 Science Parks 1 0 Saïd Business School 11 Oxford University Press PART 1 PART 1 2 The built environment 13 -

RCN International Nursing Research Conference 2017
RCN International Nursing Research Conference 2017 Wednesday 5 – Friday 7 April 2017 University of Oxford Examination Schools, 75-81 High Street, Oxford, OX1 4BG, UK Conference abstracts media partner Accrue up to 27 hours #research2017 of CPD Contents Keynote speaker abstracts 4 Concurrent session 6 54 Thursday 6 April 2017 2-2.55pm 54 Theme: Focus groups .................................54 Concurrent session 1 6 Theme: Mixed ........................................55 Wednesday 5 April 2017 11.30am-12.55pm 6 Theme: Qualitative approaches/patient safety and experience .....................................56 Theme: Qualitative approaches ..........................6 Theme: Qualitative approaches/interviews ...............57 Theme: Qualitative approaches .......................... 7 Theme: Evidence review ...............................58 Theme: Qualitative approaches ..........................8 Theme: Qualitative approaches/text and discourse ........59 Theme: Evidence review/patient safety ..................10 Theme: Questionnaires/other methods ..................60 Theme: Mixed eHealth .................................11 Theme: Research methodology ......................... 13 Concurrent session 7 62 Theme: Mixed methods/patient experience ............... 14 Friday 7 April 2017 9.50-10.45am 62 Concurrent session 2 17 Theme: Workforce/review .............................62 Wednesday 5 April 2017 1.55-3.20pm 17 Theme: Qualitative approaches .........................63 Theme: Qualitative approaches/interviewing .............64 Theme: Qualitative