From in Silico Astrocyte Cell Models to Neuron-Astrocyte Network Models: a Review Franziska Oschmann, Hugues Berry, Klaus Obermayer, Kerstin Lenk
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Deciphering the Physiology Underlying the Rapid Clinical Effects of Perispinal Etanercept in Alzheimer's Disease
Current Alzheimer Research, 2012, 9, 99-109 99 Deciphering the Physiology Underlying the Rapid Clinical Effects of Perispinal Etanercept in Alzheimer’s Disease Edward Tobinick* 100 UCLA Medical Plaza, Suites 205-210, Los Angeles, California 90095, USA Abstract: Excess tumor necrosis factor (TNF) plays a pivotal role in the pathogenesis of Alzheimer’s disease(AD). Clini- cal improvement following perispinal administration of etanercept in patients with Alzheimer’s disease and other forms of dementia and brain dysfunction is characteristically evident within minutes. The rapidity and constellation of the clinical effects across multiple domains (cognition, mood, memory, motor function, and attention) suggest they are mediated by non-synaptic signaling mechanisms previously unrecognized for etanercept. These mechanisms likely extend beyond the known roles of TNF as a gliotransmitter that modulates synaptic strength, synaptic scaling, and AMPA receptor traffick- ing. Preliminary basic science and clinical investigation suggests that perispinal administration of etanercept may lead to its rapid penetration into the cerebrospinal fluid (CSF) within the cerebral ventricles. Diffusion of large molecules into the periventricular brain parenchyma is known to occur, but this process may not be sufficient to explain the rapidity of the clinical effects. There exist populations of cells, including CSF-contacting neurons and modified ependymal cells called tanycytes, that have receptive surfaces in direct contact with the CSF. It is hypothesized that the rapid clinical effects of perispinal etanercept involve non-synaptic signal transduction across the ependymal barrier and into neuronal networks via these CSF-contacting cells. This hypothesis challenges the dogma that penetration of a therapeutic into the cerebral pa- renchyma through the endothelium of the cerebral vasculature (the so-called blood- brain barrier) is necessary to produce rapid clinical effects in AD. -

Abstract Book
Table of Contents Tuesday, May 25, 2021 ............................................................................................................................................................... 5 T1. Astrocyte-Specific Expression of the Extracellular Matrix Gene HtrA1 Regulates Susceptibility to Stress in a Sex- Specific Manner ....................................................................................................................................................................... 5 T2. Plexin-B2 Regulates Migratory Plasticity of Glioblastoma Cells in a 3D-Printed Micropattern Device ............................. 5 T3. Pathoanatomical Mapping of Differential MAPT Expression and Splicing in Progressive Supranuclear Palsy ............... 5 T4. Behavioral Variability in Response to Chronic Stress and Morphine in BXD and Parental Mouse Lines......................... 6 T5. Thyroid-Stimulating Hormone Receptor Regulates Anxiety .............................................................................................. 6 T6. Drugs That Inhibit Microglial Inflammation Also Ameliorate Aβ1-42 Induced Toxicity in C. Elegans ............................... 7 T7. Phosphodiesterase 1b is an Upstream Regulator of a Key Gene Network in the Nucleus Accumbens Driving Addiction- Like Behaviors ......................................................................................................................................................................... 7 T8. Reduced Gap Effect in Children With FOXP1 Syndrome and Autism Spectrum -

Dietary Fat Exacerbates Postprandial Hypothalamic Inflammation Involving Glial Fibrillary Acidic Protein-Positive Cells and Micr
Dietary fat exacerbates postprandial hypothalamic inflammation involving glial fibrillary acidic protein-positive cells and microglia in male mice Céline Cansell, Katharina Stobbe, Clara Sanchez, Ophélia Le Thuc, Coralie-Anne Mosser, Selma Ben-Fradj, Joris Leredde, Cynthia Lebeaupin, Delphine Debayle, Lucile Fleuriot, et al. To cite this version: Céline Cansell, Katharina Stobbe, Clara Sanchez, Ophélia Le Thuc, Coralie-Anne Mosser, et al.. Di- etary fat exacerbates postprandial hypothalamic inflammation involving glial fibrillary acidic protein- positive cells and microglia in male mice. Glia, Wiley, 2021, 69 (1), pp.42-60. 10.1002/glia.23882. hal-02899910 HAL Id: hal-02899910 https://hal.archives-ouvertes.fr/hal-02899910 Submitted on 5 Nov 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution| 4.0 International License Received: 5 December 2019 Revised: 10 June 2020 Accepted: 11 June 2020 DOI: 10.1002/glia.23882 RESEARCH ARTICLE Dietary fat exacerbates postprandial hypothalamic inflammation involving -

Astroglial Networks Scale Synaptic Activity and Plasticity
Astroglial networks scale synaptic activity and plasticity Ulrike Pannascha, Lydia Vargováb,c, Jürgen Reingruberd, Pascal Ezana, David Holcmand, Christian Giaumea, Eva Sykováb,c, and Nathalie Rouacha,1 aCenter for Interdisciplinary Research in Biology, Centre National de la Recherche Scientifique Unité Mixte de Recherche 7241/Institut National de la Santé et de la Recherche Médicale U1050, Collège de France, 75005 Paris, France; bDepartment of Neuroscience and Center for Cell therapy and Tissue Repair, Charles University, Second Faculty of Medicine, 150 06 Prague, Czech Republic; cDepartment of Neuroscience, Institute of Experimental Medicine of the Academy of Sciences of the Czech Republic, 142 20 Prague, Czech Republic; and dInstitut de Biologie de l’Ecole Normale Supérieure, Centre National de la Recherche Scientifique Unité Mixte de Recherche 8197/Institut National de la Santé et de la Recherche Médicale U1024, Department of Computational Biology and Mathematics, Ecole Normale Supérieure, Paris, 75005, France Edited* by Roger A. Nicoll, University of California, San Francisco, CA, and approved April 11, 2011 (received for review November 22, 2010) Astrocytes dynamically interact with neurons to regulate synaptic during synaptic activity. This impairment results in increased transmission. Although the gap junction proteins connexin 30 neuronal excitability, release probability, glutamate spillover, and (Cx30) and connexin 43 (Cx43) mediate the extensive network insertion of postsynaptic AMPARs, unsilencing synapses. Alto- organization of astrocytes, their role in synaptic physiology is un- gether, our findings indicate that gap junction-mediated astroglial known. Here we show, by inactivating Cx30 and Cx43 genes, that networks control synaptic strength by modulating extracellular astroglial networks tone down hippocampal synaptic transmission homeostasis. -

Specific Labeling of Synaptic Schwann Cells Reveals Unique Cellular And
RESEARCH ARTICLE Specific labeling of synaptic schwann cells reveals unique cellular and molecular features Ryan Castro1,2,3, Thomas Taetzsch1,2, Sydney K Vaughan1,2, Kerilyn Godbe4, John Chappell4, Robert E Settlage5, Gregorio Valdez1,2,6* 1Department of Molecular Biology, Cellular Biology, and Biochemistry, Brown University, Providence, United States; 2Center for Translational Neuroscience, Robert J. and Nancy D. Carney Institute for Brain Science and Brown Institute for Translational Science, Brown University, Providence, United States; 3Neuroscience Graduate Program, Brown University, Providence, United States; 4Fralin Biomedical Research Institute at Virginia Tech Carilion, Roanoke, United States; 5Department of Advanced Research Computing, Virginia Tech, Blacksburg, United States; 6Department of Neurology, Warren Alpert Medical School of Brown University, Providence, United States Abstract Perisynaptic Schwann cells (PSCs) are specialized, non-myelinating, synaptic glia of the neuromuscular junction (NMJ), that participate in synapse development, function, maintenance, and repair. The study of PSCs has relied on an anatomy-based approach, as the identities of cell-specific PSC molecular markers have remained elusive. This limited approach has precluded our ability to isolate and genetically manipulate PSCs in a cell specific manner. We have identified neuron-glia antigen 2 (NG2) as a unique molecular marker of S100b+ PSCs in skeletal muscle. NG2 is expressed in Schwann cells already associated with the NMJ, indicating that it is a marker of differentiated PSCs. Using a newly generated transgenic mouse in which PSCs are specifically labeled, we show that PSCs have a unique molecular signature that includes genes known to play critical roles in *For correspondence: PSCs and synapses. These findings will serve as a springboard for revealing drivers of PSC [email protected] differentiation and function. -
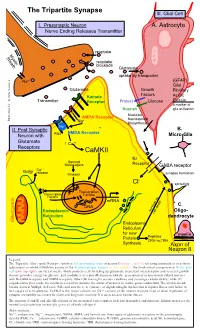
Tripartite Synapse III
The Tripartite Synapse III. Glial Cell I. Presynaptic Neuron A. Astrocyte Nerve Ending Releases Transmitter reuptake Myelin Sheath reuptake blockade Glutamate uptake by transporter Na+ (GFAP) Glial Glutamate Growth Fibrillary Factors Kainate Acidic Transmitter Receptor Protect Glucose Protein a marker of Nourish glia activation Modulate AMPA Receptor Neurosteroid Illustration by: Kendra Scouten Kendra by: Illustration Na+ Biosynthesis Mg++ II. Post Synaptic B. NMDA Receptor Neuron with PSD Micro-Glia Glutamate ↑ Ca++ Receptors CaMKII Bz Second Messengers Receptor GABA receptor ++ Golgi Ca release Kinases synapse formation - Cl sprouting Transcription Transcription Factors Factors mRNA DNA C. Endoplasmic survival vs. Oligo- cell death Reticulum dendrocyte Mitochondria Endoplasmic Reticulum for new myelin Protein Peptides CRH vs.TRH Synthesis Axon of Neuron II Legend: The Tripartite (three-part) Synapse involves: I) a presynaptic axon of neuron I (orange, top left) releasing transmitters to activate (glutamate) or inhibit (GABA-Bz) activity of the II) post-synaptic neuron (yellow, middle). The third critical component is III) the glial cell (pink, top right), an (A) astrocyte which protects cells by taking up glutamate to prevent overexcitation and secretes growth factors; provides energy via glucose; and modulates receptor (R) function with the generation of neurosteroids (which interact with Bz-GABA receptors and NMDA receptors. Other (B) microglia secrete cytokines and scavenge cellular debris; while (C) oligodendrocytes make the myelin necessary to insulate the axons of neurons to insure good conductivity. The myelin sheath breaks down in Multiple Sclerosis (MS) and now there is evidence of oligodendroglia dysfunction in bipolar illness and failure in late stages of schizophrenia. CaMK-II is the major calcium ion (Ca++) sensor of the neuron involved in up or down regulation of synaptic excitability necessary for short and long term memory. -

Neuronal Activity Determines Distinct Gliotransmitter Release from a Single Astrocyte Ana Covelo, Alfonso Araque*
RESEARCH ARTICLE Neuronal activity determines distinct gliotransmitter release from a single astrocyte Ana Covelo, Alfonso Araque* Department of Neuroscience, University of Minnesota, Minneapolis, United States Abstract Accumulating evidence indicates that astrocytes are actively involved in brain function by regulating synaptic activity and plasticity. Different gliotransmitters, such as glutamate, ATP, GABA or D-serine, released form astrocytes have been shown to induce different forms of synaptic regulation. However, whether a single astrocyte may release different gliotransmitters is unknown. Here we show that mouse hippocampal astrocytes activated by endogenous (neuron-released endocannabinoids or GABA) or exogenous (single astrocyte Ca2+ uncaging) stimuli modulate putative single CA3-CA1 hippocampal synapses. The astrocyte-mediated synaptic modulation was biphasic and consisted of an initial glutamate-mediated potentiation followed by a purinergic- mediated depression of neurotransmitter release. The temporal dynamic properties of this biphasic synaptic regulation depended on the firing frequency and duration of the neuronal activity that stimulated astrocytes. Present results indicate that single astrocytes can decode neuronal activity and, in response, release distinct gliotransmitters to differentially regulate neurotransmission at putative single synapses. DOI: https://doi.org/10.7554/eLife.32237.001 Introduction Astrocytes play key roles in brain homeostasis, such as, neurotransmitter uptake, water and ion bal- *For correspondence: ance, and blood flow control (Simard and Nedergaard, 2004; Huang et al., 2004). In addition, [email protected] astrocytes are emerging as cells with relevant roles in synaptic function, being active players of the Competing interests: The Tripartite Synapse (Araque et al., 1999). They express a wide variety of functional receptors that authors declare that no can be activated by neurotransmitters (Araque et al., 2001; Haydon and Carmignoto, 2006; competing interests exist. -

GABA As a Rising Gliotransmitter
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Frontiers - Publisher Connector REVIEW ARTICLE published: 17 December 2014 NEURAL CIRCUITS doi: 10.3389/fncir.2014.00141 GABA as a rising gliotransmitter Bo-Eun Yoon 1 and C. Justin Lee 2,3* 1 Department of Nanobiomedical Science, Dankook University, Chungnam, South Korea 2 WCI Center for Functional Connectomics, Korea Institute of Science and Technology (KIST), Seoul, South Korea 3 Center for Neural Science and Center for Functional Connectomics, Korea Institute of Science and Technology (KIST), Seoul, South Korea Edited by: Gamma-amino butyric acid (GABA) is the major inhibitory neurotransmitter that is known to Alexey Semyanov, University of be synthesized and released from GABAergic neurons in the brain. However, recent studies Nizhny Novgorod, Russia have shown that not only neurons but also astrocytes contain a considerable amount of Reviewed by: Rosemarie Grantyn, Humboldt GABA that can be released and activate GABA receptors in neighboring neurons. These University Medical School (Charité), exciting new findings for glial GABA raise further interesting questions about the source Germany of GABA, its mechanism of release and regulation and the functional role of glial GABA. In Laszlo Heja, Hungarian Academy of this review, we highlight recent studies that identify the presence and release of GABA in Sciences, Hungary glial cells, we show several proposed potential pathways for accumulation and modulation *Correspondence: C. Justin Lee, Center for Neural of glial intracellular and extracellular GABA content, and finally we discuss functional roles Science and Center for Functional for glial GABA in the brain. -

Tripartite Synapses: Glia, the Unacknowledged Partner
L ETTERS TO THE EDITOR components of the stretch-reflex system that this ‘system’ also includes the major tulated to interconnect and integrate them include: (1) dorsal-root-ganglion cells with sensory and motor systems as well. In a for whatever behavior or function is under their peripheral process that ends in stri- widely used fear-conditioning paradigm, consideration. ated-muscle stretch receptors and their auditory stimuli are used as conditioning Larry Swanson central process that ends on ventral-horn stimuli, foot-shock (somatosensory) stim- Gorica Petrovich motoneurons; and (2) the innervated uli are used as unconditioned stimuli and Neuroscience Program, University of ventral-horn motoneurons themselves. In the behavior of the animal that follows the Southern California, Los Angeles, other words, the stretch-reflex system presentation of such stimuli relies on the CA 90089-2520, USA. itself consists of parts of two classical sys- somatomotor system. In fact, very wide- tems: the somatosensory (proprioceptive) spread parts of the nervous system must References and somatomotor systems. be active during fear conditioning and 1 Lanuza, E., Martínez-Marcos, A. and Martínez-García, F. (1999) Trends Lanuza and colleagues suggest that emotional learning in general. Neurosci. 22, 207 because the basolateral amygdala and cen- Unfortunately, there is no general, 2 Nieuwenhuys, R., ten Donkellar, H.J. tral amygdala are interconnected, and have systematic theory or taxonomy of the and Nicholson, C., eds (1997) The Central Nervous System of Vertebrates, been implicated in fear conditioning and organization of mammalian neural systems. Springer-Verlag emotional learning, these two brain areas The development of one could be a major 3 Swanson, L.W. -

Glutamate Receptor Activation Triggers a Calcium- Dependent and SNARE Protein-Dependent Release of the Gliotransmitter D-Serine
Glutamate receptor activation triggers a calcium- dependent and SNARE protein-dependent release of the gliotransmitter D-serine Jean-Pierre Mothet*†, Loredano Pollegioni‡, Gilles Ouanounou*, Magalie Martineau*, Philippe Fossier*, and Ge´ rard Baux* *Laboratoire de Neurobiologie Cellulaire et Mole´ culaire, Centre National de la Recherche Scientifique Unite´ Propre de Recherche 9040, Institut Fe´de´ ratif de Neurobiologie Alfred Fessard, F-91198 Gif-sur-Yvette, France; and ‡Department of Biotechnology and Molecular Sciences, University of Insubria, 21100 Varese, Italy Edited by Solomon H. Snyder, Johns Hopkins University School of Medicine, Baltimore, MD, and approved February 25, 2005 (received for review November 19, 2004) The gliotransmitter D-serine is released upon (S)-␣-amino-3-hydroxy- increase, because efflux of the transmitter is altered by manip- 5-methyl-4-isoxazolepropionic acid͞kainate and metabotropic glu- ulations of intracellular and extracellular calcium ion concen- tamate receptor stimulation, but the mechanisms involved are trations. Furthermore, treatment of primary astrocytes or glioma unknown. Here, by using a highly sensitive bioassay to continu- cells with tetanus neurotoxin (TeNT), which selectively cleaves ously monitor extracellular D-serine levels, we have investigated the vesicle-associated SNARE proteins VAMP2 and VAMP3 the pathways used in its release. We reveal that D-serine release is (8, 9), or with concanamycin A, a compound that inhibits the inhibited by removal of extracellular calcium and augmented by vacuolar-type Hϩ-ATPase (10), strongly reduced the release of ؉ increasing extracellular calcium or after treatment with the Ca2 D-serine. Finally, we demonstrate that a large part of D-serine is ionophore A23187. Furthermore, release of the amino acid is stored in VAMP2-bearing vesicles, as revealed by sucrose- considerably reduced after depletion of thapsigargin-sensitive gradient analysis and confocal microscopy. -

Proceedings from the 2015 UK-Korea Neuroscience Symposium
www.ukorea.ac.uk/pnf PROCEEDINGS OF THE UK-KOREA NEUROSCIENCE FORUM NEW CONCEPTS OF NEUROSCIENCE FOR BASIC RESEARCH AND THERAPEUTIC ADVANCES The 8th UK-KOREA Neuroscience Symposium The Latimer Room, Clare College, Cambridge, United Kingdom 15-16 Sept. 2015 The 8th UK-KOREA Neuroscience Symposium Professor Youngchan Lee Professor Doochul Kim President of Korea Health Industry President of Institute for Basic Science Development Institute (KHIDI), (IBS), Republic of Korea Republic of Korea Dear colleagues and friends, I would like to congratulate everyone involved in hosting I would like to extend a warm welcome to everyone “The 8th UK-Korea Neuroscience Symposium”, which attending the 8th UK-Korea Neuroscience Symposium. will bring together distinguished scientists from the UK Over the past ten years UK-Korea Neuroscience and Korea. I am confident that this symposium will foster Symposia have played a vital role in bridging the gap new research relationships, and encourage our nations between neuroscience and medical research in the UK to increasingly share knowledge, ideas, and resources. and Korea, as well as being committed to ensuring our In this regard, I am delighted that the Institute for Basic research helps to address global ageing issues with Science (IBS) is actively supporting this symposium excellence and impact. since hosting last year’s at IBS Center for Synaptic Brain This symposium will feature insightful speakers from a Dysfunctions. As the president of IBS, I offer my diverse cross section of neuroscience including wholehearted support to the symposium so that we can neurodegenerative disease research. Basic, medical, and share our research outcomes and make progress in translational advancements will be highlighted, from which neuroscience research. -

Drosophila Glia: Models for Human Neurodevelopmental and Neurodegenerative Disorders
International Journal of Molecular Sciences Review Drosophila Glia: Models for Human Neurodevelopmental and Neurodegenerative Disorders Taejoon Kim, Bokyeong Song and Im-Soon Lee * Department of Biological Sciences, Center for CHANS, Konkuk University, Seoul 05029, Korea; [email protected] (T.K.); [email protected] (B.S.) * Correspondence: [email protected] Received: 31 May 2020; Accepted: 7 July 2020; Published: 9 July 2020 Abstract: Glial cells are key players in the proper formation and maintenance of the nervous system, thus contributing to neuronal health and disease in humans. However, little is known about the molecular pathways that govern glia–neuron communications in the diseased brain. Drosophila provides a useful in vivo model to explore the conserved molecular details of glial cell biology and their contributions to brain function and disease susceptibility. Herein, we review recent studies that explore glial functions in normal neuronal development, along with Drosophila models that seek to identify the pathological implications of glial defects in the context of various central nervous system disorders. Keywords: glia; glial defects; Drosophila models; CNS disorders 1. Introduction Glial cells perform many important functions that are essential for the proper development and maintenance of the nervous system [1]. During development, glia maintain neuronal cell numbers and engulf unnecessary cells and projections, correctly shaping neural circuits. In comparison, glial cells in the adult brain provide metabolic sustenance and critical immune support. Thus, the dysfunction of glial activity contributes to various central nervous system (CNS) disorders in humans at different stages of life [2]. Accordingly, the need for research regarding the initiation as well as the progression of disorders associated with glial cell dysfunction is increasing.