Stress-Induced Perinatal and Transgenerational Epigenetic
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Family Genealogy SURNAME INDEX to Date 12312015 A
Family Genealogy SURNAME INDEX to date 12312015 A A A) Misc, VF Abbey A) Abbey 1, VF Abbott A) Abbott 2, VF A) Abbott, Benj. & Augustine 1, VF W) Woodruff Genealogy (Abbott), HC* Abell A) Abell 1, VF Acker C) Descendants of Henry C. Clark (Acker), SC* Adair A) Ancestral History of Thelma D. Adair (Gander), HC Adams A) Adams 1, VF A) Adams, Abner, Zerviah 3, VF A) Adams and Griswold (Riggins), HC A) Adams Family (Adams), HC* A) Adams, Frank 2, VF H) Early Connecticut Holcomb's in Ashtabula Co., Trumbull Co., OH and PA (Holcomb), HC* R) RootAdamsMcDonaldHotling; RootHallamAtwaterGuest Genealogy (Dubach), SC W) Wright Genealogy, Moses Wright (Adams), SC Addicott A) Addicott, Beer 1, VF A) Addicott, Hersel 2, VF Addicott, James Henry Early Settler 1850, An/Cert #078, An/Cert #079 Addington Grantham & Skinner Genealogy MFM #1513336, Mfm Btm Drw Grantham & Skinner Genealogy MFM #1513337, Mfm Btm Drw Addison S) Peter Simpkins Family Genealogy (Simpkins), HC* Adset A) Adset 1, VF Aho A) Aho 1, VF G) Desendants of Casper Goodiel (Aho), SC* Aiken A) Aiken 1, VF L) Linkswilers of Louisiana (Martin), HC S) Seegar/Sager and Delp Genealogy (Williams), SC Ainger A) Ainger 1, VF Akeley A) Akeley 1, VF 1 Family Genealogy SURNAME INDEX to date 12312015 Alanko Berry, Gloucester Richard Heritage 1908, An/Cert #105 Brainard, David Pioneer 1820, An/Cert #109 Iloranta, Heikki Nestori Heritage 1919, An/Cert #106 I) The Iloranta and Soukka Families in America (Alanko), SC K) Klingman Family History (Alanko), SC* Albert A) Albert 1, VF Alden A) Alden, David 1, VF Alderman A) Alderman 1, VF A) Alderman 2, VF A) Alderman 3, VF A) Aldermans in America (Parker), SC A) Descendants of William Alderman. -

1 Epigenetic Regulation and Fetal Programming
Best Practice & Research Clinical Endocrinology & Metabolism Vol. 22, No. 1, pp. 1–16, 2008 doi:10.1016/j.beem.2007.07.009 available online at http://www.sciencedirect.com 1 Epigenetic regulation and fetal programming Christine Gicquel* MD, PhD Doctor Epigenetics in Human Health and Disease, Baker Medical Research Institute, 75 Commercial Road, Melbourne, 3004 Victoria, Australia Assam El-Osta PhD Associate Professor Epigenetics in Human Health and Disease, Baker Medical Research Institute, 75 Commercial Road, Melbourne, 3004 Victoria, Australia Yves Le Bouc MD, PhD Professor APHP-UPMC, Hoˆpital Armand Trousseau, Laboratoire d’Explorations Fonctionnelles Endocriniennes; INSERM U.515, Paris, France Fetal programming encompasses the role of developmental plasticity in response to environmental and nutritional signals during early life and its potential adverse consequences (risk of cardiovascu- lar, metabolic and behavioural diseases) in later life. The first studies in this field highlighted an as- sociation between poor fetal growth and chronic adult diseases. However, environmental signals during early life may lead to adverse long-term effects independently of obvious effects on fetal growth. Adverse long-term effects reflect a mismatch between early (fetal and neonatal) environ- mental conditions and the conditions that the individual will confront later in life. The mechanisms underlying this risk remain unclear. However, experimental data in rodents and recent observa- tions in humans suggest that epigenetic changes in regulatory genes and growth-related genes play a significant role in fetal programming. Improvements in our understanding of the biochemical and molecular mechanisms at play in fetal programming would make it possible to identify bio- markers for detecting infants at high risk of adult-onset diseases. -

Magazin Wirtschaft
www.stuttgart.ihk.de 07.2013 Stuttgart - Böblingen - Esslingen-Nürtingen - Göppingen - Ludwigsburg - Rems-Murr Magazin Wirtschaft Ein Service der IHK für Unternehmen in der Region Stuttgart Fachkräfte finden zwischen Alb und Gäu Seite 6 wer hat schon ein ganzes kabinett nur für diewirtschaft? Selbstverständlich wir. Damit die Betriebe gute Rahmenbedingungen haben, sind in unseren Gremien ausschließlich Wirtschaftexperten vertreten. www.stuttgart.ihk.de oder Infoline 0711 2005-0 EDITORIAL Handelskriege kennen keine Gewinner Ähnlich dem Frühjahrswetter in diesem weit kommen? China ist die zweitgrößte Wirt- Jahr ziehen nun auch Wolken über dem schaftsmacht der Welt. Daher sollte das Land Parkett des internationalen Handels beginnen, sich an internationale Spielregeln auf. Auslöser ist die vorläufige Erhebung von zu halten. Globalisierung benötigt faire Wett- EU-Strafzöllen auf chinesische Solarmodule. bewerbsbedingungen, an die sich China zu- China kontert mit einem Anti-Dumping-Ver- weilen jedoch nicht hält. Belegte Fälle von fahren gegen europäischen Wein. Immer Wettbewerbsverstößen gibt es genug. mehr Produkte treten in den Fokus der Dum- ping-Diskussion. Vernünftige Handelspolitik Die EU muss schnellstens sieht anders aus. Mangels Deeskalation wird Verhandlungen aufnehmen die Situation für China und Europa immer tassilo zywietz unangenehmer. Ein Handelskrieg droht. Europa darf davor die Augen nicht ver- Geschäftsführer International Globalisierung erfordert jedoch gegensei- schließen. Nachgiebigkeit ist keine Erfolg ver- der IHK Region Stuttgart tige Rücksichtnahme und Handeln im Sinne sprechende Strategie – damit würde die EU der immer stärker miteinander verbundenen erpressbar. Lässt man China gewähren, kann Volkswirtschaften: China und die EU haben die Methode künftig in der Chemiebranche letztes Jahr Waren und Dienstleistungen in und irgendwann im Autobau angewendet Höhe von über 400 Milliarden Euro ausge- werden. -

2013 Kaiser Permanente-Authored Publications Alphabetical by Author
2013 Kaiser Permanente-Authored Publications Alphabetical by Author 1. Abbas MA, Cannom RR, Chiu VY, Burchette RJ, Radner GW, Haigh PI, Etzioni DA Triage of patients with acute diverticulitis: are some inpatients candidates for outpatient treatment? Colorectal Dis. 2013 Apr;15(4):451-7. Southern California 23061533 KP author(s): Abbas, Maher A; Chiu, Vicki Y; Burchette, Raoul J; Radner, Gary W; Haigh, Philip I 2. Abbass MA, Slezak JM, DiFronzo LA Predictors of Early Postoperative Outcomes in 375 Consecutive Hepatectomies: A Single- institution Experience Am Surg. 2013 Oct;79(10):961-7. Southern California 24160779 KP author(s): Abbass, Mohammad Ali; Slezak, Jeffrey M; DiFronzo, Andrew L 3. Abbenhardt C, Poole EM, Kulmacz RJ, Xiao L, Curtin K, Galbraith RL, Duggan D, Hsu L, Makar KW, Caan BJ, Koepl L, Owen RW, Scherer D, Carlson CS, Genetics and Epidemiology of Colorectal Cancer Consortium (GECCO) and CCFR, Potter JD, Slattery ML, Ulrich CM Phospholipase A2G1B polymorphisms and risk of colorectal neoplasia Int J Mol Epidemiol Genet. 2013 Sep 12;4(3):140-9. Northern California 24046806 KP author(s): Caan, Bette 4. Aberg JA, Gallant JE, Ghanem KG, Emmanuel P, Zingman BS, Horberg MA Primary Care Guidelines for the Management of Persons Infected With HIV: 2013 Update by the HIV Medicine Association of the Infectious Diseases Society of America Clin Infect Dis. 2013 Nov 13. Mid-Atlantic 24235263 KP author(s): Horberg, Michael 5. Abraham AG, D'Souza G, Jing Y, Gange SJ, Sterling TR, Silverberg MJ, Saag MS, Rourke SB, Rachlis A, Napravnik S, Moore RD, Klein MB, Kitahata MM, Kirk GD, Hogg RS, Hessol NA, Goedert JJ, Gill MJ, Gebo KA, Eron JJ, Engels EA, Dubrow R, Crane HM, Brooks JT, Bosch RJ, Strickler HD, North American AIDS Cohort Collaboration on Research and Design of IeDEA Invasive cervical cancer risk among HIV-infected women: A North American multi-cohort collaboration prospective study J Acquir Immune Defic Syndr. -

Family Group Sheets Surname Index
PASSAIC COUNTY HISTORICAL SOCIETY FAMILY GROUP SHEETS SURNAME INDEX This collection of 660 folders contains over 50,000 family group sheets of families that resided in Passaic and Bergen Counties. These sheets were prepared by volunteers using the Societies various collections of church, ceme tery and bible records as well as city directo ries, county history books, newspaper abstracts and the Mattie Bowman manuscript collection. Example of a typical Family Group Sheet from the collection. PASSAIC COUNTY HISTORICAL SOCIETY FAMILY GROUP SHEETS — SURNAME INDEX A Aldous Anderson Arndt Aartse Aldrich Anderton Arnot Abbott Alenson Andolina Aronsohn Abeel Alesbrook Andreasen Arquhart Abel Alesso Andrews Arrayo Aber Alexander Andriesse (see Anderson) Arrowsmith Abers Alexandra Andruss Arthur Abildgaard Alfano Angell Arthurs Abraham Alje (see Alyea) Anger Aruesman Abrams Aljea (see Alyea) Angland Asbell Abrash Alji (see Alyea) Angle Ash Ack Allabough Anglehart Ashbee Acker Allee Anglin Ashbey Ackerman Allen Angotti Ashe Ackerson Allenan Angus Ashfield Ackert Aller Annan Ashley Acton Allerman Anners Ashman Adair Allibone Anness Ashton Adams Alliegro Annin Ashworth Adamson Allington Anson Asper Adcroft Alliot Anthony Aspinwall Addy Allison Anton Astin Adelman Allman Antoniou Astley Adolf Allmen Apel Astwood Adrian Allyton Appel Atchison Aesben Almgren Apple Ateroft Agar Almond Applebee Atha Ager Alois Applegate Atherly Agnew Alpart Appleton Atherson Ahnert Alper Apsley Atherton Aiken Alsheimer Arbuthnot Atkins Aikman Alterman Archbold Atkinson Aimone -

Surname Folders.Pdf
SURNAME Where Filed Aaron Filed under "A" Misc folder Andrick Abdon Filed under "A" Misc folder Angeny Abel Anger Filed under "A" Misc folder Aberts Angst Filed under "A" Misc folder Abram Angstadt Achey Ankrum Filed under "A" Misc folder Acker Anns Ackerman Annveg Filed under “A” Misc folder Adair Ansel Adam Anspach Adams Anthony Addleman Appenzeller Ader Filed under "A" Misc folder Apple/Appel Adkins Filed under "A" Misc folder Applebach Aduddell Filed under “A” Misc folder Appleman Aeder Appler Ainsworth Apps/Upps Filed under "A" Misc folder Aitken Filed under "A" Misc folder Apt Filed under "A" Misc folder Akers Filed under "A" Misc folder Arbogast Filed under "A" Misc folder Albaugh Filed under "A" Misc folder Archer Filed under "A" Misc folder Alberson Filed under “A” Misc folder Arment Albert Armentrout Albight/Albrecht Armistead Alcorn Armitradinge Alden Filed under "A" Misc folder Armour Alderfer Armstrong Alexander Arndt Alger Arnold Allebach Arnsberger Filed under "A" Misc folder Alleman Arrel Filed under "A" Misc folder Allen Arritt/Erret Filed under “A” Misc folder Allender Filed under "A" Misc folder Aschliman/Ashelman Allgyer Ash Filed under “A” Misc folder Allison Ashenfelter Filed under "A" Misc folder Allumbaugh Filed under "A" Misc folder Ashoff Alspach Asper Filed under "A" Misc folder Alstadt Aspinwall Filed under "A" Misc folder Alt Aston Filed under "A" Misc folder Alter Atiyeh Filed under "A" Misc folder Althaus Atkins Filed under "A" Misc folder Altland Atkinson Alwine Atticks Amalong Atwell Amborn Filed under -

Sales Report
Sales Listing Report Page 1 of 309 McHenry County 05/04/2020 09:46:13 01/01/2019 - 12/31/2019 Township: GRAFTON TWP Document Number Sale Year Sale Type Valid Sale Sale Date Dept. Study Selling Price Parcel Number Built Year Property Type Prop. Class Acres Square Ft. Lot Size Grantor Name Grantee Name Site Address 2019R0006019 2019 Not advertised on mN 02/19/2019 N $115,000.00 WILLIAM MCDONALD PETER J. GODLEWSKI DOLORESS MCDONALD 78 S HEATHER DR 18-01-101-013 0 GARAGE/ NO O O 0040 .00 0 CRYSTAL LAKE, IL 600145018 78 S HEATHER DR CRYSTAL LAKE, IL 60014 -5018 Legal Description: DOC 2019R0006019 LT 16 & 17 BLK 15 R A CEPEKS CRYSTAL VISTA 2019R0027941 2019 Not advertised on mN 07/08/2019 N $150,000.00 CHARLES G. ALEMAN REINVEST HOMES LLC, AN ILLINOIS LIMIT DAWN S. ALEMAN 503 E ALGONQUIN RD 18-01-101-027 0 GARAGE/ NO O O 0040 .00 0 ALGONQUIN, IL 601023004 160 HEATHER DR CRYSTAL LAKE, IL 60014 - Legal Description: DOC 2019R0027941 LT 31 BLK 15 R A CEPEKS CRYSTAL VISTA 2019R0026844 2019 Warranty Deed Y 08/14/2019 Y $172,000.00 THOMAS M. COFFMAN JESSICA N. NICHOLAS KATRINA COFFMAN 1350 THORNWOOD LN 18-01-102-035 0 LDG SINGLE FAM 0040 .00 0 CRYSTAL LAKE, IL 600145042 1350 THORNWOOD LN CRYSTAL LAKE, IL 60014 -5042 Legal Description: DOC 2019R0026844 LT 6 & PT LT 19 LYING N OF & ADJ BLK 14 R A CEPEKS CRYSTAL VISTA 2019R0010406 2019 Warranty Deed Y 04/12/2019 Y $174,000.00 JEREMY M. -

Andrew Thomas Kerr Joint Honours MA (Arts) 2Nd Upper
Kerr, Andrew Thomas (2009) The significance of the Wigtownshire Hearth Tax lists. MPhil(R) thesis. http://theses.gla.ac.uk/2786/ Copyright and moral rights for this thesis are retained by the author A copy can be downloaded for personal non-commercial research or study, without prior permission or charge This thesis cannot be reproduced or quoted extensively from without first obtaining permission in writing from the Author The content must not be changed in any way or sold commercially in any format or medium without the formal permission of the Author When referring to this work, full bibliographic details including the author, title, awarding institution and date of the thesis must be given Glasgow Theses Service http://theses.gla.ac.uk/ [email protected] The significance of the Wigtownshire Hearth Tax lists Andrew Thomas Kerr Joint Honours MA (Arts) 2nd Upper Submitted in fulfilment of the requirements of the Degree of MPhil Department of Scottish History Faculty of Arts University of Glasgow October 2009 1 Abstract Presentation of the 1695 Wigtownshire Hearth Tax edition together with a thesis focussing on the historical value of the tax lists. The discussion provides a historical context for the tax lists and includes an analysis of the distribution of hearths, kilns, smiddies, saltpans and furnaces as indicators of wealth, social status and evidence of social, economic and agricultural development. Comparison is provided with other Hearth Tax lists and with contemporary records such as the poll tax returns, and also from later records such as early census information. The Hearth Tax is also compared with different Wigtownshire records from earlier and later periods (Wigtownshire Charters, parish records and the statistical accounts). -

Most-Common-Surnames-Bmd-Registers-16.Pdf
Most Common Surnames Surnames occurring most often in Scotland's registers of Births, Marriages and Deaths Counting only the surname of the child for births, the surnames of BOTH PARTIES (for example both BRIDE and GROOM) for marriages, and the surname of the deceased for deaths Note: the surnames from these registers may not be representative of the surnames of the population of Scotland as a whole, as (a) they include the surnames of non-residents who were born / married / died here; (b) they exclude the surnames of residents who were born / married / died elsewhere; and (c) some age-groups have very low birth, marriage and death rates; others account for most births, marriages and deaths.ths Registration Year = 2016 Position Surname Number 1 SMITH 2056 2 BROWN 1435 3 WILSON 1354 4 CAMPBELL 1147 5 STEWART 1139 6 THOMSON 1127 7 ROBERTSON 1088 8 ANDERSON 1001 9 MACDONALD 808 10 TAYLOR 782 11 SCOTT 771 12 REID 755 13 MURRAY 754 14 CLARK 734 15 WATSON 642 16 ROSS 629 17 YOUNG 608 18 MITCHELL 601 19 WALKER 589 20= MORRISON 587 20= PATERSON 587 22 GRAHAM 569 23 HAMILTON 541 24 FRASER 529 25 MARTIN 528 26 GRAY 523 27 HENDERSON 522 28 KERR 521 29 MCDONALD 520 30 FERGUSON 513 31 MILLER 511 32 CAMERON 510 33= DAVIDSON 506 33= JOHNSTON 506 35 BELL 483 36 KELLY 478 37 DUNCAN 473 38 HUNTER 450 39 SIMPSON 438 40 MACLEOD 435 41 MACKENZIE 434 42 ALLAN 432 43 GRANT 429 44 WALLACE 401 45 BLACK 399 © Crown Copyright 2017 46 RUSSELL 394 47 JONES 392 48 MACKAY 372 49= MARSHALL 370 49= SUTHERLAND 370 51 WRIGHT 357 52 GIBSON 356 53 BURNS 353 54= KENNEDY 347 -

Electrophysiology Read-Out Tools for Brain-On-Chip Biotechnology
micromachines Review Electrophysiology Read-Out Tools for Brain-on-Chip Biotechnology Csaba Forro 1,2,†, Davide Caron 3,† , Gian Nicola Angotzi 4,†, Vincenzo Gallo 3, Luca Berdondini 4 , Francesca Santoro 1 , Gemma Palazzolo 3,* and Gabriella Panuccio 3,* 1 Tissue Electronics, Fondazione Istituto Italiano di Tecnologia, Largo Barsanti e Matteucci, 53-80125 Naples, Italy; [email protected] (C.F.); [email protected] (F.S.) 2 Department of Chemistry, Stanford University, Stanford, CA 94305, USA 3 Enhanced Regenerative Medicine, Fondazione Istituto Italiano di Tecnologia, Via Morego, 30-16163 Genova, Italy; [email protected] (D.C.); [email protected] (V.G.) 4 Microtechnology for Neuroelectronics, Fondazione Istituto Italiano di Tecnologia, Via Morego, 30-16163 Genova, Italy; [email protected] (G.N.A.); [email protected] (L.B.) * Correspondence: [email protected] (G.P.); [email protected] (G.P.); Tel.: +39-010-2896-884 (G.P.); +39-010-2896-493 (G.P.) † These authors contributed equally to this paper. Abstract: Brain-on-Chip (BoC) biotechnology is emerging as a promising tool for biomedical and pharmaceutical research applied to the neurosciences. At the convergence between lab-on-chip and cell biology, BoC couples in vitro three-dimensional brain-like systems to an engineered microfluidics platform designed to provide an in vivo-like extrinsic microenvironment with the aim of replicating tissue- or organ-level physiological functions. BoC therefore offers the advantage of an in vitro repro- duction of brain structures that is more faithful to the native correlate than what is obtained with conventional cell culture techniques. -
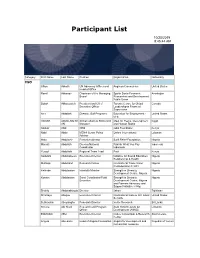
Participant List
Participant List 10/20/2019 8:45:44 AM Category First Name Last Name Position Organization Nationality CSO Jillian Abballe UN Advocacy Officer and Anglican Communion United States Head of Office Ramil Abbasov Chariman of the Managing Spektr Socio-Economic Azerbaijan Board Researches and Development Public Union Babak Abbaszadeh President and Chief Toronto Centre for Global Canada Executive Officer Leadership in Financial Supervision Amr Abdallah Director, Gulf Programs Educaiton for Employment - United States EFE HAGAR ABDELRAHM African affairs & SDGs Unit Maat for Peace, Development Egypt AN Manager and Human Rights Abukar Abdi CEO Juba Foundation Kenya Nabil Abdo MENA Senior Policy Oxfam International Lebanon Advisor Mala Abdulaziz Executive director Swift Relief Foundation Nigeria Maryati Abdullah Director/National Publish What You Pay Indonesia Coordinator Indonesia Yussuf Abdullahi Regional Team Lead Pact Kenya Abdulahi Abdulraheem Executive Director Initiative for Sound Education Nigeria Relationship & Health Muttaqa Abdulra'uf Research Fellow International Trade Union Nigeria Confederation (ITUC) Kehinde Abdulsalam Interfaith Minister Strength in Diversity Nigeria Development Centre, Nigeria Kassim Abdulsalam Zonal Coordinator/Field Strength in Diversity Nigeria Executive Development Centre, Nigeria and Farmers Advocacy and Support Initiative in Nig Shahlo Abdunabizoda Director Jahon Tajikistan Shontaye Abegaz Executive Director International Insitute for Human United States Security Subhashini Abeysinghe Research Director Verite -

Exposure to Smoke During Development: Fetal Programming of Adult Disease
TOBACCO INDUCED DISEASES Vol. 3, No. 2:5-16 (2006) © PTID Society Exposure to Smoke During Development: Fetal Programming of Adult Disease Hugo T. Bergen Dept. of Human Anatomy & Cell Science, University of Manitoba, ABSTRACT: It is well established that smoking has potent effects on a number of parameters including food intake, body weight, metabolism, and blood pressure. For example, it is well documented that 1) there is an inverse relationship between smoking and body weight, and 2) smoking cessation is associated with weight gain. However, there is increasing evidence that smoking can exert deleterious effects on energy balance through maternal exposure during fetal development. Specifically, there appears to be an increased incidence of metabolic disease (including obesity), and cardiovascular disease in children and adults that were exposed to smoke during fetal development. The present review will examine the relationship between maternal smoke and adult disease in offspring. The epidemiological studies highlighting this relationship will be reviewed as well as the experimental animal models that point to potential mechanisms underlying this relationship. A better understanding of how smoking effects changes in energy balance may lead to treatments to ameliorate the long-lasting effects of perinatal exposure to smoke as well as increasing the health benefits associated with smoking cessation. Smoking And The Fetal Origins Of and newborn growth and the subsequent Disease development of obesity is a commonly cited example of this