Using the Δ18 O-Salinity Relationship to Identify
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Geological History of Southwestern Nova Scotia - C
CH MINING RAN MATTERS B REPORT ME 2004-2 2004 ED. D. R. MACDONALD ESOURCES ALR INER THE WESTIN NOVA SCOTIAN HOTEL NOVEMBER 1 AND 2, 2004 M Natural Resources Honourable Richard Hurlburt Minister Peter Underwood Deputy Minister Halifax, Nova Scotia 2004 019621 ii CONTENTS Program ....iv Peat Resources of Southwestern Nova Scotia - A. R. Anderson ....1 The Callie Lode Gold Deposit, Northern Territory, Australia: High- grade, Sheeted, Auriferous Quartz Veins in an Anticlinal Structural Environment - J. Bigelow and C. R. Stanley .....2 Geology and Economic Potential of Upper Carboniferous Rocks in Nova Scotia: Tradition Meets Innovation - J. H. Calder ..... 3 A Comparison of the Late Triassic Dinosaur Footprints Atreipus acadianus and Grallator (Grallator) sp. from the Upper Wolfville Formation at North Medford, Nova Scotia - B. Cameron and N. Wood ...4 A Geological and Geophysical Study of the Large, Positive Magnetic Anomaly between Eastern Prince Edward Island and Western Cape Breton Island, Nova Scotia - L. Cook, S. M. Barr and S. Dehler ....5 The Story of Halifax Harbour - G. B. J. Fader ..... 6 Recent Advances in the Geology of the Windsor Group in Nova Scotia - P. S. Giles ... 7 Update on the Bedrock Mapping Component of the Targeted Geoscience Initiative (Phase 2) - P. S. Giles, R. D. Naylor and D. C. Brisco ....8 Nova Scotia Gold Grain Study: Background Concentrations and Distance of Transport - T. A. Goodwin ...... 9 Regional and Detailed Geochemical Sampling of Humus, Soil and Till in the Upper and Lower Seal Harbour Gold Districts - T. A. Goodwin . .... 10 Regional Humus, Soil and Till Sampling for the Targeted Geoscience Initiative (Phase 2) - T. -

Convoy Cup Mini-Offshore Race September 12, 2020
Notice of Race Convoy Cup Mini-Offshore Race September 12, 2020 1. Organizing Authority: These races are hosted by the Dartmouth Yacht Club of Dartmouth, Nova Scotia. 2. Objectives: The Convoy Cup Ocean Race offers racing and cruising yachts an opportunity to participate in an ocean race to commemorate the links that formed between the province of Nova Scotia and the countries of Europe during the two world wars. Halifax was the congregation point for hundreds of naval vessels and supply ships that formed convoys to transport the necessities of life across the Atlantic Ocean; this race is dedicated to the memory of all those men and women in the navy and merchant marine service who sailed in those convoys. 3. Rules: Racing will be governed by the Racing Rules of Sailing 2017-2020 (RRS), the prescriptions of the Canadian Yachting Association and this Notice of Race except as modified by the Sailing Instructions. Dartmouth Yacht Club Race Committee (RC) will have final authority on all matters. 4. Description: Normally the Convoy Cup is an overnight 100 n/m ocean race and a Basin Race is also held. This season is quite different due to the COVID 19 pandemic so the event this year has been changed to a mini-offshore race. The Convoy Cup Mini-Offshore Race will be comprised of 1 race of approximately 30 n/m (course and distances may be adjusted according to forecast winds and conditions). 5. Start date, course and finish: The races will commence September 12, 2020 at 1200 at a start line established between the Navy Island buoy HY2 (Mark 11on the DYC course card) and the RC flag on the Race Committee boat passing either side of George’s Island outbound only, and proceeding to HB, port rounding and return keeping George’s Island to starboard, to finish at the CSS Acadia dock, at in a line projected from the edge of the wharf, which is closest to the Last Steps Memorial. -

They Planted Well: New England Planters in Maritime Canada
They Planted Well: New England Planters in Maritime Canada. PLACES Acadia University, Wolfville, Nova Scotia, 9, 10, 12 Amherst Township, Nova Scotia, 124 Amherst, Nova Scotia, 38, 39, 304, 316 Andover, Maryland 65 Annapolis River, Nova Scotia, 22 Annapolis Township, Nova Scotia, 23, 122-123 Annapolis Valley, Nova Scotia, 10, 14-15, 107, 178 Annapolis County, Nova Scotia, 20, 24-26, 28-29, 155, 258 Annapolis Gut, Nova Scotia, 43 Annapolis Basin, Nova Scotia, 25 Annapolis-Royal (Port Royal-Annapolis), 36, 46, 103, 244, 251, 298 Atwell House, King's County, Nova Scotia, 253, 258-259 Aulac River, New Brunswick, 38 Avon River, Nova Scotia, 21, 27 Baie Verte, Fort, (Fort Lawrence) New Brunswick, 38 Barrington Township, Nova Scotia, 124, 168, 299, 315, Beaubassin, New Brunswick (Cumberland Basin), 36 Beausejour, Fort, (Fort Cumberland) New Brunswick, 17, 22, 36-37, 45, 154, 264, 277, 281 Beaver River, Nova Scotia, 197 Bedford Basin, Nova Scotia, 100 Belleisle, Annapolis County, Nova Scotia, 313 Biggs House, Gaspreau, Nova Scotia, 244-245 Blomidon, Cape, Nova Scotia, 21, 27 Boston, Massachusetts, 18, 30-31, 50, 66, 69, 76, 78, 81-82, 84, 86, 89, 99, 121, 141, 172, 176, 215, 265 Boudreau's Bank, (Starr's Point) Nova Scotia, 27 Bridgetown, Nova Scotia, 196, 316 Buckram (Ship), 48 Bucks Harbor, Maine, 174 Burton, New Brunswick, 33 Calkin House, Kings County, 250, 252, 259 Camphill (Rout), 43-45, 48, 52 Canning, Nova Scotia, 236, 240 Canso, Nova Scotia, 23 Cape Breton, Nova Scotia, 40, 114, 119, 134, 138, 140, 143-144 2 Cape Cod-Style House, 223 -

New Brunswick and Nova Scotia:: the First Geological Field
Document généré le 2 oct. 2021 13:35 Atlantic Geology New Brunswick and Nova Scotia: the First Geological Field Trip by a North American College Howard J. Falcon-Lang Volume 45, 2009 Résumé de l'article La première excursion géologique connue d’un collège nord‑américain a été URI : https://id.erudit.org/iderudit/ageo45art05 réalisée en 1835. Vingt membres du personnel et étudiants du Collège Williams — collège d’arts libéraux du Massachusetts, Etats-Unis — ont exploré la Aller au sommaire du numéro géologie des bords de la baie de Fundy dans le nord‑est du Maine, au Nouveau‑Brunswick et en Nouvelle‑Écosse. Le groupe dirigé par deux jeunes professeurs d’histoire naturelle, Ebenezer Emmons et Albert Hopkins, a Éditeur(s) effectué de nombreuses observations dans les environs de la baie de Passamaquoddy, de Saint‑Jean, de Parrsboro et de Windsor, ainsi que dans des Atlantic Geoscience Society secteurs plus étendus à l’intérieur des bassins Minas et Cumberland. Même si le groupe du Collège Williams a en partie suivi les pas de deux pionniers de ISSN Boston, Charles Jackson et Francis Alger, qui avaient effectué une reconnaissance de la région vers la fin des années 1820, il a néanmoins fait 0843-5561 (imprimé) plusieurs observations originales. L’une des plus importantes a été l’étude de 1718-7885 (numérique) l’anatomie et de l’importance paléoclimatique des végétaux minéralisés de Joggins et de l’île Grindstone réalisée par Emmons. Il s’agissait seulement de la Découvrir la revue deuxième étude du genre à l’échelle mondiale; elle a ultérieurement inspiré Williams Dawson à exécuter des travaux similaires. -

Using Oxygen Isotopes to Establish Freshwater Sources in Bedford Basin, Nova Scotia, a Northwestern Atlantic Fjord
Estuarine, Coastal and Shelf Science 199 (2017) 96e104 Contents lists available at ScienceDirect Estuarine, Coastal and Shelf Science journal homepage: www.elsevier.com/locate/ecss Using oxygen isotopes to establish freshwater sources in Bedford Basin, Nova Scotia, a Northwestern Atlantic fjord * Elizabeth A. Kerrigan, Markus Kienast, Helmuth Thomas, Douglas W.R. Wallace Dalhousie University, Department of Oceanography, 1355 Oxford Street, PO Box 15000, B3H 4R2, Halifax, NS, Canada article info abstract Article history: A weekly time-series of oxygen isotope (d18O) measurements was collected over a 16-month period from Received 10 March 2017 near-surface (1 m) and near-bottom (60 m) waters of Bedford Basin, a coastal fjord adjacent to the Available online 8 September 2017 Scotian Shelf, off eastern Canada. The time-series was complemented with d18O measurements of local precipitation (rain and snow), river, and wastewater runoff. The isotopic composition of precipitation Keywords: displayed strong seasonality with an average (volume-weighted) d18O value of À5.39‰ (±0.96) for Bedford Basin summer and a depleted value of À10.37‰ (±2.96) over winter. Winter precipitation exhibited more Fjord depleted and variable d18O of solid precipitation relative to rainfall. The annual, amount-weighted Oxygen isotopes d18 À ‰ ± Salinity average O of Sackville River discharge ( 6.49 0.82) was not statistically different from precipi- À ‰ ± Seasonality tation ( 7.24 0.92), but exhibited less seasonal variation. Freshwater end-members (zero-salinity 18 Freshwater inputs intercepts) estimated from annual and seasonal regressions of d O versus salinity (S) for Bedford Basin near-surface samples were consistent with the d18O of summer precipitation and the annual, amount- weighted average for the Sackville River. -

Sackville Rivers Floodplain Study: Phase I Final Report
Sackville Rivers Floodplain Study: Phase I Final Report Halifax Regional Municipality 45 Akerley Boulevard Dartmouth Nova Scotia B3B 1J7 11102282 | Report No 4 | October 30 2015 October 30, 2015 Reference No. 11102282-4 Mr. Cameron Deacoff Halifax Regional Municipality PO Box 1749 Halifax, NS B3J 3A5 Dear Mr. Deacoff: Re: Sackville Rivers Floodplain Study: Phase I Final Report GHD is pleased to provide the Halifax Regional Municipality (HRM) with the attached Final Report for the Sackville Rivers Floodplain Study: Phase I. This report presents the final results for this study, including: flood and sea level frequency analyses, joint flood and sea level probability analysis, hydraulic modelling, topo-bathymetric survey data collection, and analysis of flooding factors. Data sources, methodology, and results are described in detail. Recommendations for the Phase II Study are also provided. All of which is respectfully submitted, GHD Yours truly, Juraj M. Cunderlik, Ph.D., P.Eng. Prof. Edward McBean, Ph.D., P.Eng. Project Manager QA/QC Reviewer Allyson Bingeman, Ph.D., P.Eng. Andrew Betts, M.A.Sc., P.Eng. Statistical Hydrology Specialist Hydraulic Modelling Specialist JC/jp/3 Encl. GHD Limited 45 Akerley Boulevard Dartmouth Nova Scotia B3B 1J7 Canada T 902 468 1248 F 902 468 2207 W www.ghd.com Executive Summary The lower reaches of the Sackville River have been the site of several instances of flooding over the last decade, which has been a significant issue for the Halifax Regional Municipality (HRM). A Hydrotechnical Study of the Sackville River was performed in 1981, and a Hydrotechnical Study of the Little Sackville River delineated the floodplain in 1987. -

The Maritime Aspects of the 1917 Halifax Explosion Joseph Scanlon
Source of Threat and Source of Assistance: The Maritime Aspects of the 1917 Halifax Explosion Joseph Scanlon The 1917 Halifax explosion is usually thought of as an event that devastated the cities of Halifax and Dartmouth. It is true that most of the 1963 dead and 9000 injured were civilians - the largest single group of dead were Roman Catholics and/or housewives - and the greatest damage was to private homes in the Halifax's North End. But the explosion resulted from a fire caused by a collision between a Norwegian and a French ship. It damaged or destroyed four British merchant ships - Calonne, Curaca, Middleham Castle and Picton - and a Norwegian vessel (Hovland). It caused substantial damage to the British cruiser, High Flyer, a US Coast Guard vessel, Morill; three tugs, Hilford, Stella Maris and Wasper B; and another smaller vessel, Raguse. It also destroyed a schooner, San Bernardo. It left Halifax harbour so badly damaged that convoys were delayed, some incoming traffic was re-routed and the Admiralty changed the rules for North Atlantic shipping. After the explosion, sailors from British, Canadian and American ships in harbour helped in search and rescue, and teamed up with civilians to use boats to transport the injured along the waterfront.1 A US Navy ship, Old Colony, became a floating hospital. In addition, two other USN vessels, Tacoma and Von Steuben, arrived in Halifax five and one-half hours after the explosion. For eight days, they assisted the Canadian Army with security. After reviewing the situation in Halifax harbour prior to the explosion, this article looks at the collision that led to the fire and eventually to the explosion. -

Assessment of Rbrcoda T.ODO Performance on Long-Term
Assessment of RBRcoda T.ODO performance on long- term deployment and profiling in Bedford Basin 1 Executive summary........................................................................................................1 2 Why Bedford Basin? .......................................................................................................1 3 Instruments and deployments......................................................................................2 4 Data post-processing.....................................................................................................3 5 Results ............................................................................................................................4 6 Conclusions ....................................................................................................................6 7 References......................................................................................................................7 8 Appendix.........................................................................................................................7 1 Executive summary The RBRcoda T.ODO is an optical dissolved oxygen (DO) sensor with a standard accuracy of 8 μmol/l and a power consumption of only 36 mJ/sample. It is available in three variants, T.ODO|slow (30 s τ), T.ODO|standard (8 s τ), and T.ODO|fast (1 s τ), providing oceanographers more options for applications, such as long-term deployments, vertical profiling, and integration on gliders. To assess the performance of the RBRcoda -

Program and Abstracts
Atlantic Geoscience Society ABSTRACTS 2004 Colloquium & Annual General Meeting Moncton, New Brunswick The 2004 Colloquium & Annual General Meeting was held at the Delta Beausejour Hotel, Moncton, New Brunswick, on January 30 and 31, 2004. On behalf of the society, we thank Colloquium Chairperson Susan Johnson and her organizing committee (Alan Anderson, Cameron Bartsch, Robin Black, Lori Cook, Russell Hiebert, Ken Howells, Dave Keighley, Maurice Mazerolle, Tansy O'Connor-Parsons, Michael Parkhill, Brian Roulston, Erin Smith, Ian Spooner, Peter Wallace, and Reg Wilson) for providing an excellent meeting. We also wish to acknowledge support of the corporate sponsors: PCS-Potash, New Brunswick Division; McGregor GeoScience Limited; Mineralogical Association of Canada; Corridor Resources Inc.; and the Dean of Science, St. Francis Xavier University. In the following pages, we are pleased to publish the abstracts of oral presentations and posters from the Colloquium, which focused on The First J. Ewart Blanchard Special Session on Geophysics; Environmental Geology; Hydrocarbon Geology; Current Research in the Atlantic Provinces; an Ion Microprobe Workshop; and a tour of the PCS Potash Mine. THE EDITORS Trial This PDF was created using the Free RoboPDF Trial (for evaluation purposes only!) Version Get RoboPDF: An Easy, Affordable Alternative for Creating PDFs - www.robopdf.com Buy RoboPDF Contrasting behaviour of acid-generating rock of the Meguma Supergroup in fresh and salt water Jennifer Arnold, Stephen Armstrong, and Anne Marie O’Beirne-Ryan Department of Earth Sciences, Dalhousie University, Halifax, NS B3H 3J5, Canada Acid rock drainage is a well known problem associated with the Meguma Supergroup in the Halifax region of Nova Scotia, Canada. -

The Collision of the SS Mont-Blanc and the Halifax Explosion
National Aeronautics and Space Administration NASA SAFETY CENTER SYSTEM FAILURE CASE STUDY JANUARY 2013 VOLUME 7 ISSUE 1 Kiloton Killer PROXIMATE CAUSE The Collision of the SS Mont-Blanc and the Halifax Explosion • The Imo collided with the Mont- December 6, 1917, Halifax, Nova Scotia, Canada: the Great War reached across the Atlantic to Blanc, rupturing containers of transform one of North America’s natural deepwater ports into a teeming logistics hub. In benzol on the Mont-Blanc’s deck. a new tactic, the Allies formed shipping convoys against lurking German U-boats to deliver Sparks from the grinding metal tons of supplies. To join one such convoy, the French freighter SS Mont-Blanc entered Halifax hulls ignited barrels of benzene which, in turn, set off the Mont- Harbour that bright sunny afternoon. Known only to the crew and a few port officials, the ship Blanc’s explosive cargo. was packed with munitions. Steaming outbound, the SS Imo collided with the Mont-Blanc. UNDERLYING ISSUES Sparks set the French ship on fire beyond the crew’s ability to extinguish the flames; they escaped and the ship erupted into the largest explosion yet created by man. Blast pressure • Procedures Fail leveled every structure within a mile and blew wreckage for tens of miles. Approximately • Poor Communication 2,000 people were killed and over 9,000 injured. The unprecedented recovery effort that AFTERMATH followed was the genesis of modern disaster relief programs. • The large numbers of disciplined men stationed at Halifax greatly expedited immediate disaster response and later recovery. BACKGROUND in advent of German U-boat strikes • Disaster relief supplies and money on shipping crossing the Atlantic. -

Bedford Institute of Oceanography
BEDFORD INSTITUTE OF OCEANOGRAPHY ANNUAL REVIEW I 2005 Change of address notices, requests for copies, and other correspondence regarding this publication should be sent to: The Editor, BIO 2005 in Review Bedford Institute of Oceanography P.O. Box 1006 Dartmouth, Nova Scotia Canada B2Y 4A2 E-mail address: [email protected] Cover photo: The CCGS Amundsen passes through Lancaster Sound during the ship’s inaugural voyage in August 2003, almost 100 years to the day after Roald Amundsen sailed the same waters during his historic voyage through the Northwest Passage on the Gjoa (1903-1906). © Martin Fortier, ArcticNet NCE Photo is courtesy of Dr. Martin Fortier, who was Chief Scientist on the voyage. Dr. Fortier, of Université Laval, is the Executive Director of ArcticNet, a Network of Centres of Excellence of Canada that brings together scientists and managers in the natural, human health, and social sciences with their partners in Inuit organizations, northern communities, government, and industry to help Canadians face the impacts and opportunities of climate change in the Arctic. Back cover photo: CCGS Amundsen engaged in mapping work in Canada’s Arctic, August 2005 © Jonathan Beaudoin Photo is courtesy of Jonathan Beaudoin, a PhD student at the University of New Brunswick in Fredericton, whose main research interest is the application of oceanographic databases for multibean echosounding in the Canadian Arctic Archipelago. Jonathan, who holds bachelor degrees in Geomatics Engineering and Computer Science, is the Ocean Mapping Group’s dedicated -
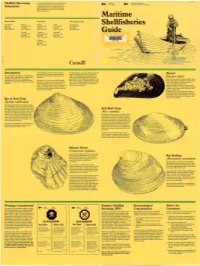
Shellfish Harvesting Information
Shellfish Harvesting It is very important before you col lect any shell Fishe ries Environmen t Canada fish that you ensure the area is not closed . and Oceans Environmental Protection Service Information A check is as simple as using the local tele phone directory to find the federal fisheries •• •• office nearest you or ca ll one of the central offices listed below. Maritime New Brunswick Nova Scotia Prince Edward Island St. Andrews Moncion Sydney Halifax Charlottetown Shellfisheries Box 210 P.O . Box 5030 P.O. Box 1085 P 0 . Box 550 P.O. Box 1236 EOG 2XO E1C 9B6 B1 p 6J7 B3J 2S7 C1A 7M8 (506) 529-8847 (506) 758-9044 (902) 564-7276 (902) 426-24 73 (902) 566-7800 Tracadie Yarmouth Antigonish Guide .,. P.O. Box 1670 215 Main St reet P.O. Box 1183 EOC 2BO B5A 1C6 B2G 2M5 (506) 395-6321 (902) 742-9122 (902) 863-5670 Liverpool P.O. Box 190 BOT 1KO (902) 354-3459 Canada Introduction Fresh shellfish can be purchased at any of As with an y food , care must be taken to ensure hundreds of fish markets , and are served at th at the shellfish gathered are not contam Mussel One of the great attractions of Canada's Mari restaurants and snack bars throughout the inated. This guide has been prepared to pro time provinces is the selection of seafood del Maritimes. vide the recreational digger with information (Mytilus edulis) icacies to be enjoyed here. Among the pertaining to the safe harvest of shellfish in the Rocky shores along the three provinces' coast favourites are the many varieties of shellfish In search of a recreational outing ? Shellfish Maritimes.