Comparative Geographic Variation of Selected Southern African Gerbils
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
PLAGUE STUDIES * 6. Hosts of the Infection R
Bull. Org. mond. Sante 1 Bull. World Hlth Org. 1952, 6, 381-465 PLAGUE STUDIES * 6. Hosts of the Infection R. POLLITZER, M.D. Division of Epidemiology, World Health Organization Manuscript received in April 1952 RODENTS AND LAGOMORPHA Reviewing in 1928 the then rather limited knowledge available concerning the occurrence and importance of plague in rodents other than the common rats and mice, Jorge 129 felt justified in drawing a clear-cut distinction between the pandemic type of plague introduced into human settlements and houses all over the world by the " domestic " rats and mice, and " peste selvatique ", which is dangerous for man only when he invades the remote endemic foci populated by wild rodents. Although Jorge's concept was accepted, some discussion arose regarding the appropriateness of the term " peste selvatique" or, as Stallybrass 282 and Wu Lien-teh 318 translated it, " selvatic plague ". It was pointed out by Meyer 194 that, on etymological grounds, the name " sylvatic plague " would be preferable, and this term was widely used until POzzO 238 and Hoekenga 105 doubted, and Girard 82 denied, its adequacy on the grounds that the word " sylvatic" implied that the rodents concerned lived in forests, whereas that was rarely the case. Girard therefore advocated the reversion to the expression "wild-rodent plague" which was used before the publication of Jorge's study-a proposal it has seemed advisable to accept for the present studies. Much more important than the difficulty of adopting an adequate nomenclature is that of distinguishing between rat and wild-rodent plague- a distinction which is no longer as clear-cut as Jorge was entitled to assume. -

How Will Climate Change Affect the Temporal and Spatial Distributions Of
Evolutionary Ecology Research, 2018, 19: 215–226 How will climate change affect the temporal and spatial distributions of a reservoir host, the Indian gerbil (Tatera indica), and the spread of zoonotic diseases that it carries? Kordiyeh Hamidi1, Saeed Mohammadi2 and Naeimeh Eskandarzadeh3 1Department of Biology, Faculty of Science, Ferdowsi University of Mashhad, Mashhad, Iran, 2Department of Environmental Sciences, Faculty of Natural Resources, University of Zabol, Zabol, Iran and 3Young Researchers and Elite Club, Islamic Azad University, Shirvan Branch, Shirvan, Iran ABSTRACT Background: The Indian gerbil (Tatera indica) is a main reservoir host of cutaneous leish- maniasis, a great public health problem in many rural areas of Iran. Questions: How do climatic variables affect the habitat suitability and distribution of T. indica? How will changes in climatic variables affect the spatial distribution of T. indica across Iran? Will those changes influence the outbreak regions of zoonotic cutaneous leishmaniasis? Organism: The Indian gerbil, T. indica, a rodent. Analytical methods: Maximum entropy modelling (MaxEnt) to predict suitable regions and the potential distribution of this gerbil in the present and future in Iran. Results: Species distribution models revealed the four variables most effective in determining Indian gerbil occurrence: the mean precipitation of the year’s driest month; the seasonality of precipitation; the mean temperature of the warmest quarter of the year; and the mean temperature of the wettest quarter. According to our model, the southern parts of Iran have the most suitable habitat for T. indica. With global climate change, suitable habitats for the gerbil will increase considerably in Iran spreading outwards toward the southwest, centrally, and the northeast. -

Reproductive Behaviour of the South Indian Gerbil Tat Era Indie a Cuvieri with a Note on the Role of Postejaculatory Copulations
Acta Theriologica 43 (3): 263-270, 1998. PL IiSSN 0001-7051 Reproductive behaviour of the South Indian gerbil Tat era indie a cuvieri with a note on the role of postejaculatory copulations Biju B. THOMAS and Mathew M. OOMMEN Thomas B. B. and Oommen M. M. 1998. Reproductive behaviour of the South Indian gerbil Tatera indica cuvieri with a note on the role of postejaculatory copulations. Acta Theriologica 43: 263-270. The reproductive behaviour of Tatera indica cuvieri (Waterhouse, 1838) has been evaluated in detail. It involves 1 to 4 series, with each series composed of mounts, intromissions, and ending up with ejaculation. Quantitative measures of the copulatory behavioural variables indicate significant difference across the 4 series. While intro- mission duration and thrust frequency progressively increased across the 4 series, significant decrease was observed regarding postejaculatory interval. After the final ejaculation, the male resorted to a state of continued copulatory activity, referred to as postejaculatory copulation (PEC). Higher levels of copulatory activity are observed during this phase as evidenced by increases in total number of intromissions, duration of intromissions and thrust frequency. Further, the postejaculatory copulation is of considerable significance in inducing pregnancy in females, the chance of pregnancy being higher if the female is subjected to PEC. Department of Zoology, University of Kerala, Kariavattom 695 581, Trivandrum, Kerala State, India Key words'. Tatera indica cuvieri, copulatory behaviour, postejaculatory copulation, pregnancy Introduction The drive to reproduce is one of life's strongest imperatives. This is accompli- shed by adult male and female behavioural patterns that have evolved to ensure fertilization and survival of the young. -

A Re-Evaluation of Allometric Relationships for Circulating Concentrations of Glucose in Mammals
Food and Nutrition Sciences, 2016, 7, 240-251 Published Online April 2016 in SciRes. http://www.scirp.org/journal/fns http://dx.doi.org/10.4236/fns.2016.74026 A Re-Evaluation of Allometric Relationships for Circulating Concentrations of Glucose in Mammals Colin G. Scanes Department of Biological Science, University of Wisconsin Milwaukee, Milwaukee, WI, USA Received 10 August 2015; accepted 19 April 2016; published 22 April 2016 Copyright © 2016 by author and Scientific Research Publishing Inc. This work is licensed under the Creative Commons Attribution International License (CC BY). http://creativecommons.org/licenses/by/4.0/ Abstract Purpose: The present study examined the putative relationship between circulating concentra- tions of glucose and log10 body weight in a large sample size (270) of wild species but with domes- ticated animals excluded from the analyses. Methods: A data-set of plasma/serum concentration of glucose and body weight in mammalian species was developed from the literature. Allometric re- lationships were examined. Results: In contrast to previous reports, no overall relationship for circulating concentrations of glucose was observed across 270 species of mammals (for log10 glu- cose concentration adjusted R2 = −0.003; for glucose concentration adjusted R2 = −0.003). In con- trast, a strong allometric relationship was observed for circulating concentrations of glucose in 2 Primates (for log10 glucose concentration adjusted R = 0.511; for glucose concentration adjusted R2 = 0.480). Conclusion: The absence of an allometric relationship for circulating concentrations of glucose was unexpected. A strong allometric relationship was seen in Primates. Keywords Glucose, Allometric, Mammals, Primates 1. Introduction Glucose in the blood is the principal energy source for brain functioning and but glucose can be used as the energy source for multiple other tissues. -

Rodent Control in India
Integrated Pest Management Reviews 4: 97–126, 1999. © 1999 Kluwer Academic Publishers. Printed in the Netherlands. Rodent control in India V.R. Parshad Department of Zoology, Punjab Agricultural University, Ludhiana 141004, India (Tel.: 91-0161-401960, ext. 382; Fax: 91-0161-400945) Received 3 September 1996; accepted 3 November 1998 Key words: agriculture, biological control, campaign, chemosterilent, commensal, control methods, economics, environmental and cultural methods, horticulture, India, pest management, pre- and post-harvest crop losses, poultry farms, rodent, rodenticide, South Asia, trapping Abstract Eighteen species of rodents are pests in agriculture, horticulture, forestry, animal and human dwellings and rural and urban storage facilities in India. Their habitat, distribution, abundance and economic significance varies in different crops, seasons and geographical regions of the country. Of these, Bandicota bengalensis is the most predominant and widespread pest of agriculture in wet and irrigated soils and has also established in houses and godowns in metropolitan cities like Bombay, Delhi and Calcutta. In dryland agriculture Tatera indica and Meriones hurrianae are the predominant rodent pests. Some species like Rattus meltada, Mus musculus and M. booduga occur in both wet and dry lands. Species like R. nitidus in north-eastern hill region and Gerbillus gleadowi in the Indian desert are important locally. The common commensal pests are Rattus rattus and M. musculus throughout the country including the islands. R. rattus along with squirrels Funambulus palmarum and F. tristriatus are serious pests of plantation crops such as coconut and oil palm in the southern peninsula. F. pennanti is abundant in orchards and gardens in the north and central plains and sub-mountain regions. -

The Changing Rodent Pest Fauna in Egypi'
THE CHANGING RODENT PEST FAUNA IN EGYPI' A. MAHER ALI. Plant Protection Department Asslut University. Asslut. Egypt ABSTRACT: The most serious known rodent pests in agricultural irrigated land are: Rattus rattus. Arvicanthis niloticus and Acomys sp. Occasionally there are rodent outbreaks in agricultural planta tions. The changing agro-ecosystem in the present and future agricultural plantations is expected to affect the status of the following potential rodent pest species: Spalax ehrenbergi aegyptiacus, Nesokia indica, Jaculus oriental is, and Gerbillus gerbillus gerbillus. Basic studies are needed to quantify damage including water loss, which is caused by rodents, forecast of rodent outbreaks. and integrated control of rodents in agricultural projects. INTRODUCTION The depredations of rodents and the struggle to prevent them will never diminish, even though man desires to raise his standard of living and health. In the face of the rapidly rising human population in Egypt, the problem has become more acute. The pattern of rodent populations is changed when man converts deserts, forests and rangeland into food or fiber production schemes. This is a corrmon feature of the Middle East region, including Egypt. In some cases such activities are carried out without prior knowledge of the actual fauna of the area, and the natural predators of rodents are driven away , or killed for food, or for the sake of their skins. As a result rodents increase in number and different rodent species may appear. The causes of shifts in species distribution are mostly due to changes in environmental conditions and the superior survival strategy of the replacing species. There are several examples of a predominant rodent species being replaced by another one under various ecological conditions. -

Water Requirements of Desert Ungulates Desert Ungulates Requirements of Water Literature Review and Annotated Bibliography: Review and Annotated Literature
Resources U.S. Department of the Interior U.S. Geological Survey Southwest Biological Science Center Open-File Report 2005-1141 April 2005 In Cooperation with the University of Arizona School of Natural and Arizona Game and Fish Department Water Requirements of Desert Ungulates Water Literature Review and Annotated Bibliography: g r a p n s& t o T c u k r n e r v i e w a n C ain III, Kra usm an, Rose Literature R e d A nnotated Biblio O pen-File Report 2005-1141 hy: W ater Require m ents of D esert U ngulates In cooperation with the University of Arizona School of Natural Resources and Arizona Game and Fish Department Literature Review and Annotated Bibliography: Water Requirements of Desert Ungulates By James W. Cain III, Paul R. Krausman, Steven S. Rosenstock, and Jack C. Turner Open-File Report 2005-1141 April 2005 USGS Southwest Biological Science Center Sonoran Desert Research Station University of Arizona U.S. Department of the Interior School of Natural Resources 125 Biological Sciences East U.S. Geological Survey Tucson, Arizona 85721 U.S. Department of the Interior Gale A. Norton, Secretary U.S. Geological Survey Charles G. Groat, Director U.S. Geological Survey, Reston, Virginia: 2005 Note: This document contains information of a preliminary nature and was prepared primarily for internal use in the U.S. Geological Survey. This information is NOT intended for use in open literature prior to publication by the investigators named unless permission is obtained in writing from the investigators named and from the Station Leader. -

A Study of Small Mammals Inhabiting Pistachio Gardens of Kerman Province, Southeast Iran
BIHAREAN BIOLOGIST 7 (1): pp.13-19 ©Biharean Biologist, Oradea, Romania, 2013 Article No.: 131102 http://biozoojournals.3x.ro/bihbiol/index.html A study of small mammals inhabiting pistachio gardens of Kerman Province, Southeast Iran Seyed Massoud MADJDZADEH1,* and Haji Mohammad TAKALLOOZADEH1,2 1. Department of Biology, Faculty of Sciences, Shahid Bahonar University of Kerman, Kerman 76169-14111, Iran. E-mail: [email protected] 2. Department of Plant protection, Faculty of Agriculture, Shahid Bahonar University of Kerman, Kerman 76169-14111, Iran. E-mail: [email protected] *Corresponding author, S.M. Madjdzadeh, E-mail: [email protected] Received: 21. April 2012 / Accepted: 26. February 2013 / Available online: 3. March 2013 / Printed: June 2013 Abstract. This study was performed in order to indentify the rodent fauna inhabiting pistachio gardens in Kerman Province, Southeast Iran. In this order we used live trapping method for the sampling of rodents. In total, 105 rodent specimens were collected. The specimens were studied in respect to their morphological, cranial and external characteristics. The results showed that six species belonging to five genera of rodents and one species of lagomorpha occur in this region. The identified samples were as fallow: Cricetulus migratorius, Mus musculus, Nesokia indica, Meriones persicus, Meriones libycus, Tatera indica, and Ochotona rufescenc. Mus musculus and Cricetulus migratorius had maximum and minimum abundance respectively. Morphological and morphometric characteristics of the identified species were investigated. Key words: Rodentia, Lagomorpha, Pistachio gardens, Kerman Province, Iran. Introduction some rodents in the agricultural areas of Southern parts of Kerman Province (Missone 1990). To date no such studies Rodents constitute one of the largest orders of mammals, are carried out in agricultural areas such as pistachio gar- which composed of about 29 families, 443 genera and 2277 dens in Kerman Province. -

Ecology of Small Mammals of Desert and Montane Ecosystems
ECOLOGY OF SMALL MAMMALS OF DESERT AND MONTANE ECOSYSTEMS Ishwar Prakash Ph.D., D.Sc. Emeritus Professor of Eminence Desert Regional Station, Zoological Survey of India Jodhpur - 342 005 (India) and Partap Singh M.Phil, Ph.D. Department of Zoology, Govt. Dungar College, BIKANER - 334 001 (India) SCIENTIFIC PUBLISHERS (INDIA) P.O. BOX 91 JODHPUR Published by: PAWAN KUMAR SCIENTIFIC PUBLISHERS (INDIA) 5-A, New Pali Road, P.O. Box 91 JODHPUR - 342 001 (Raj.) E-mail: [email protected] www.scientificpub.com © Prakash & Singh, 2005 ISBN: 81-7233-401-X Laser typeset : Rajesh Ojha Printed in India …. a note With great humility and submission I take this opportunity to thank Prof. Prakash for imparting knowledge and training to me in the field of ecology and ethology. Prof. Prakash, popularly known as IP among friends and colleagues, was an eminent scientist, great academician and above all a prefect gentleman. When he selected me for "Aravallis Project", I had confused state of mind as I had cytogenetics background in M.Sc. and M.Phil. Will I be able to justify change of subject, and will it be feasible for me to carry out arduous fieldwork were a few questions boggling my mind. After discussing the things with scientists of Zoological Survey of India, where Prof. Prakash was working as Senior Scientist of Indian National Science Academy, I was convinced to join the project. While trapping small mammals in the jungles of Aravallis, he asked us to keep our eyes open and make notes of what so ever animal we come across. -
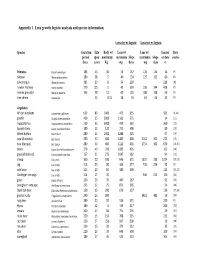
Appendix 1. Lens Growth Logistic Analysis and Species Information
Appendix 1. Lens growth logistic analysis and species information. Lens dry wt logistic Lens wet wt logistic Species Gestation Life Body wt Lens wt Lens wt Lenses Data period span maximum maximum Slope maximum Slope or data source days years Kg mg days mg days n Primates Papio hamadryas 183 45 30 55 167 178 124 16 PS baboon Macaca fascicularis 180 38 5 40 130 125 162 60 PS cynomolgus Alouatta caraya 185 20 8 34 220 228 [8] howler monkey Homo sapiens 270 115 5 45 359 198 244 608 PS human prenatal Macaca mulatta 165 38 12 60 125 188 108 50 PS tree shrew Tupaia glis 12 0.15 28 76 63 63 32 PS Ungulates African elephant Loxodonta a africana 659 80 5000 475 935 561 [9,10] giraffe Giraffa camelopardalis 460 35 2000 1163 571 34 [11] hipppotamus Hippopotamus amphibius 250 55 3000 410 362 360 [12] Spanish Ibex Capra pyrenaica Schinz 180 12 120 711 508 80 [13] Wood buffalo Bison bison 280 25 1000 1288 525 97 [14] cow (Australia) Bos taurus 280 30 600 1483 400 3162 302 231 [15] cow (Europe) Bos taurus 280 30 400 1122 401 2754 302 630 [16,17] zebra Equus burchelli antiquorum 370 40 350 1085 436 102 [18] gnu (wildebeest) Connochaetes taurinus 257 21 275 1047 362 94 [19] sheep Ovis aries 160 20 100 646 311 1622 239 1224 [20,21] pig Sus scrofa 115 25 80 364 277 736 254 92 PS wild boar Sus scrofa 115 20 84 389 309 113 [22,23] Gottingen minipigs Sus scrofa 114 17 35 646 211 100 [24] goat Capra hircus L 150 20 70 447 267 91 [25] pronghorn antelope Antilocapra americana 235 12 75 871 365 24 [26] black tail deer Odocoileus hemionus columbianus 150 15 200 -

A C T a T H E R I O L O G I
ACTA THERIOLOGICA VOL. 20, 10: 123—132. April, 1975 B. D. R A N A, A. P. J A I N & Ishwar P R A K A S H Morphological Variation in the Gerbils Inhabiting the Indian Desert |With 7 Tables] The paper deals with a comparison of external body parts and cranial measurements of two Gerbils, Gerbillus gleadowi. Murray, 1886 and G. nanus indus (Thomas, 1920) collected from various localities in the Indian desert. Inter-population comparisons have been made. Male G. gleadowi are found to be larger than females in all the populations. G. gleadowi collected at Jhunjhunu, receiving highest amount of precip- itation (430 mm) are largest in head and body size and those from Sind (91 mm) are smallest. However, the tail and ear lengths of gerbils from localities bear an inverse relationship with the aridity index. Various body parts take a midposition in gerbils collected from Bikaner (291 mm). The data presented suggests that body size of the same species decreases with the increasing aridity, whereas tail and ear lengths in- crease with the increasing aridity. The various external body parts and cranial measurements in male and female G. n. indus do not differ sig- nificantly. The tail, hind foot and ear lengths in the Sind specimens are significantly larger than those of the Rajasthan material. I. INTRODUCTION A comparison was made of external body parts and cranial measurem- ents in two population of the Indian Gerbil, Tatera indica indica H a r - dwicke, 1807 inhabiting different types of habitats in the Indian desert (R a n a et al., 1970). -

Accounts of Nepalese Mammals and Analysis of the Host-Ectoparasite Data by Computer Techniques Richard Merle Mitchell Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1977 Accounts of Nepalese mammals and analysis of the host-ectoparasite data by computer techniques Richard Merle Mitchell Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Zoology Commons Recommended Citation Mitchell, Richard Merle, "Accounts of Nepalese mammals and analysis of the host-ectoparasite data by computer techniques " (1977). Retrospective Theses and Dissertations. 7626. https://lib.dr.iastate.edu/rtd/7626 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS This material was produced from a microfilm copy of the original document. While the most advanced technological means to photograph and reproduce this document have been used, the quality s heavily dependent upon the quality of the original submitted. The following explanation of techniques is provided to help you understand rrarkings or patterns which may appear on this reproduction. 1. The sign or "target" for pages apparently lacking from the document photographed is "Missing Page(s)". If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting thru an image and duplicating adjacent pages to insure you complete continuity. 2. When an image on the film is obliterated with a large round black mark, it is an indication that the photographer suspected that the copy may have moved during exposure and thus cause a blurred image.