Commercial Musculoskeletal Codes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Post–Vertebral Augmentation Back Pain: ORIGINAL RESEARCH Evaluation and Management
Post–Vertebral Augmentation Back Pain: ORIGINAL RESEARCH Evaluation and Management S. Kamalian BACKGROUND AND PURPOSE: Vertebral augmentation is an established treatment for painful osteo- R. Bordia porotic vertebral fractures of the spine. Nevertheless, patients may continue to have significant back pain afterward. The purpose of this study was to assess the source of persistent or recurrent back pain A.O. Ortiz following vertebral augmentation. MATERIALS AND METHODS: Our institutional review board approved this study. We evaluated 124 consecutive patients who underwent vertebral augmentation for painful osteoporotic vertebral frac- tures. All patients were evaluated after 3 weeks, 3 months, and 1 year following their procedure. Patients with any type of back pain after their procedure were examined under fluoroscopy. RESULTS: Thirty-four of 124 (27%) patients were men, and 90/124 (73%) were women. Persistent or recurrent back pain, not due to a new fracture or a failed procedure, was present in 29/124 (23%) patients. The source of pain was most often attributed to the sacroiliac and/or lumbar facet joints (25/29 or 86%). Seventeen of 29 (59%) patients experienced immediate relief after facet joint injection of a mixture of steroid and local anesthetic agents. The remaining 12 (41%) had relief after additional injections. Ten (34%) patients ultimately required radio-frequency neurolysis for long-term relief. CONCLUSIONS: Back pain after vertebral augmentation may not be due to a failed procedure but rather to an old or a new pain generator, such as an irritated sacroiliac or lumbar facet joint. This is of importance not only for further pain management of these patients but also for designing trials to compare the efficacy of vertebral augmentation to other treatments. -

Procedure Codes
NEW YORK STATE MEDICAID PROGRAM ORDERED AMBULATORY PROCEDURE CODES Ordered Ambulatory Procedure Codes Table of Contents GENERAL INFORMATION ---------------------------------------------------------------------------------------------------------------2 LABORATORY SERVICES INFORMATION-----------------------------------------------------------------------------------------2 RADIOLOGY INFORMATION------------------------------------------------------------------------------------------------------------3 MMIS MODIFIERS --------------------------------------------------------------------------------------------------------------------------6 RADIOLOGY SERVICES------------------------------------------------------------------------------------------------------------------7 DIAGNOSTIC RADIOLOGY (DIAGNOSTIC IMAGING) ------------------------------------------------------------------------7 DIAGNOSTIC ULTRASOUND SERVICES- -------------------------------------------------------------------------------------- 20 RADIOLOGIC GUIDANCE....................................................................................................................................25 BREAST, MAMMOGRAPHY --------------------------------------------------------------------------------------------------------- 26 BONE/JOINT STUDIES --------------------------------------------------------------------------------------------------------------- 26 RADIATION ONCOLOGY SERVICES --------------------------------------------------------------------------------------------- 27 NUCLEAR -

Vertebral Augmentation ICD 9 Codes: Osteoporosis 733. 0, Vertebra
BRIGHAM AND WOMEN’S HOSPITAL Department of Rehabilitation Services Physical Therapy Standard of Care: Vertebral Augmentation ICD 9 Codes: Osteoporosis 733. 0, Vertebral Fracture closed 805.8, Pathological fracture of Vertebrae 733.13 Vertebral augmentation, known as vertebroplasty and kyphoplasty, is a minimally invasive procedure that is used to treat vertebral fractures. Vertebral fractures are the most common skeletal injury associated with osteoporosis, and it is estimated that more than 750,000 occur annually in the United States.1 Up to one quarter of people over 50 years of age will have at least one vertebral fracture in their life time secondary to osteoporosis.2 According to the World Health Organization (WHO), the operational definition of osteoporosis is a bone density measure >2.5 standard deviations (SD) below the mean of young healthy adults of similar race and gender.3 Primary osteoporosis is related to the changes in postmenopausal women secondary to reduction of estrogen levels and related to age-related loss of bone mass. Secondary osteoporosis is the loss of bone caused by an agent or disease process. 1,4 (See Osteoporosis SOC) The severity of vertebral fractures can be assessed by the Genat semiquantitative method. Commonly used by radiologists, this scale assesses the severity of the fracture visually and has been shown to be reliable.5 Genat Semiquantitive Grading System for Vertebral Deformity5 Grade 0- normal vertebral height Grade 1- minimal fracture- 20-25% height decrease Grade 2- moderate fracture- 25-40% height decrease Grade 3-severe- >40% height decrease Standard methods of diagnosing vertebral fractures are imaging, including the following: CT scan, MRI, and radiography. -
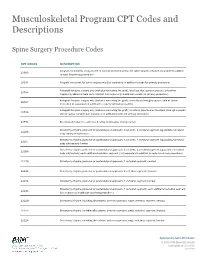
Musculoskeletal Program CPT Codes and Descriptions
Musculoskeletal Program CPT Codes and Descriptions Spine Surgery Procedure Codes CPT CODES DESCRIPTION Allograft, morselized, or placement of osteopromotive material, for spine surgery only (List separately in addition 20930 to code for primary procedure) 20931 Allograft, structural, for spine surgery only (List separately in addition to code for primary procedure) Autograft for spine surgery only (includes harvesting the graft); local (eg, ribs, spinous process, or laminar 20936 fragments) obtained from same incision (List separately in addition to code for primary procedure) Autograft for spine surgery only (includes harvesting the graft); morselized (through separate skin or fascial 20937 incision) (List separately in addition to code for primary procedure) Autograft for spine surgery only (includes harvesting the graft); structural, bicortical or tricortical (through separate 20938 skin or fascial incision) (List separately in addition to code for primary procedure) 20974 Electrical stimulation to aid bone healing; noninvasive (nonoperative) Osteotomy of spine, posterior or posterolateral approach, 3 columns, 1 vertebral segment (eg, pedicle/vertebral 22206 body subtraction); thoracic Osteotomy of spine, posterior or posterolateral approach, 3 columns, 1 vertebral segment (eg, pedicle/vertebral 22207 body subtraction); lumbar Osteotomy of spine, posterior or posterolateral approach, 3 columns, 1 vertebral segment (eg, pedicle/vertebral 22208 body subtraction); each additional vertebral segment (List separately in addition to code for -

Effectiveness of Cementoplasty for Vertebral Augmentation in Multiple Myeloma: a Case Series
WCRJ 2017; 4 (2): e882 EFFECTIVENESS OF CEMENTOPLASTY FOR VERTEBRAL AUGMENTATION IN MULTIPLE MYELOMA: A CASE SERIES G. TESTA1, M. PRIVITERA1, T. FIDILIO1, G. DI STEFANO1, A. VESCIO1, G. D’ANGELO2, V. PAVONE1 1Department of Orthopedics and Traumatologic Surgery, AOU Policlinico-Vittorio Emanuele, University of Catania, Catania, Italy 2Department of Human Pathology in Adult and Developmental Age “Gaetano Barresi” – Unit of Paediatrics, University of Messina, Messina, Italy Abstract – Objective: Multiple myeloma (MM) is a neoplasm characterized by the proliferation of somatically mutated plasma cells that tend to expand within the bone marrow and affect mul- tiple locations throughout the bone marrow. When it is located in vertebral areas it causes bone lesions with pain, kyphosis, walking impairments, and disability. Different types of treatments are available. The goal of this study is to report our experience regarding the treatment of vertebral fractures from multiple myeloma using cementoplasty. Patients and Methods: From January 2012 to December 2015, 38 patients with multiple mye- loma and multilevel vertebral fractures were treated. Seventeen patients underwent conservative treatment (group 1), and 21 patients underwent vertebral augmentation with percutaneous ce- mentoplasty (group 2). Both groups were clinically evaluated at 1, 6 and 12 months using a visual analogic scale (VAS) for pain, SF-36 and ODI Score Questionnaires. Radiographic evaluation was performed to verify the quality of cementoplasty and complications. Results: Mean follow-up was 23.7 months. Mean VAS score in group 1 decreased from 7.1 pre-operatively to 3.9 at final follow-up (p<0.05). In group 2, this score decreased from 7.3 pre-op- eratively to 2.3 at final follow-up (p<0.05). -

Clinical Guidelines
CLINICAL GUIDELINES Interventional Pain Management Services Version 1.0.2019 Clinical guidelines for medical necessity review of comprehensive musculoskeletal management services. © 2019 eviCore healthcare. All rights reserved. Regence: Comprehensive Musculoskeletal Management Guidelines V1.0.2019 Interventional Pain Management CMM-200: Epidural Steroid Injections (ESI) 3 CMM-201: Facet Joint Injections/Medial Branch Blocks 17 CMM-202: Trigger Point Injections 21 CMM-203: Sacroiliac Joint Injections 32 CMM-204: Prolotherapy 37 CMM-207: Epidural Adhesiolysis 40 CMM-208: Radiofrequency Joint Ablations/Denervations 44 CMM-209: Regional Sympathetic Blocks 51 CMM 210: Implantable Intrathecal Drug Delivery Systems 57 CMM-211: Spinal Cord Stimulators 65 CMM-308: Thermal Intradiscal Procedures 66 CMM-310: Manipulation of the Spine Under Anesthesia 71 ______________________________________________________________________________________________________ © 2019 eviCore healthcare. All Rights Reserved. Page 2 of 73 400 Buckwalter Place Boulevard, Bluffton, SC 29910 (800) 918-8924 www.eviCore.com Regence: Comprehensive Musculoskeletal Management Guidelines V1.0.2019 CMM-200: Epidural Steroid Injections (ESI) CMM-200.1: Definitions 4 CMM-200.2: General Guidelines 5 CMM-200.3: Indications: Selective Nerve Root Block (SNRB) 6 CMM-200.4: Indications: Epidural Steroid Injections 7 CMM-200.5: Non-Indications: SNRB 8 CMM-200.6: Non-Indications: ESI 8 ® CMM-200.7: Procedure (CPT ) Codes 9 CMM-200.8: References 10 ______________________________________________________________________________________________________ -

TESSYS Technique with Small Grade of Facetectomy Has Potential Biomechanical Advantages Compared to the In-Out TED with Intact Articular Process : an In-Silico Study
TESSYS Technique With Small Grade of Facetectomy Has Potential Biomechanical Advantages Compared to the In-Out TED With Intact Articular Process : An In-Silico Study Jingchi Li West China Hospital/West China School of Medicine for Sichuan University Chen Xu Changzheng Hospital Aliated to the Naval Medical University Xiaoyu Zhang Aliated Hospital of Integrated Traditional Chinese and Western Medicine for Nanjing University of Chinese Medicine Zhipeng Xi Aliated Hospital of Integrated Traditional Chinese and Western Medicine for Nanjing University of Chinese Medicine Mengnan Liu Macau University of Science and Technology Zhongxin Fang Xihua University Nan Wang Aliated Hospital of Integrated Traditional Chinese and Western Medicine for Nanjing University of Chinese Medicine Lin Xie ( [email protected] ) Aliated Hospital of Integrated Traditional Chinese and Western Medicine for Nanjing University of Chinese Medicine Yueming Song West China Hospital/West China School of Medicine for Sichuan University Research Article Keywords: Biomechanical deterioration, Transforaminal endoscopic discectomy, Endoscopic dynamic drill, Facetectomy, Iatrogenic annulus injury Posted Date: April 26th, 2021 Page 1/27 DOI: https://doi.org/10.21203/rs.3.rs-429749/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Version of Record: A version of this preprint was published at BMC Musculoskeletal Disorders on July 10th, 2021. See the published version at https://doi.org/10.1186/s12891-021-04504-1. Page 2/27 Abstract Background: The facetectomy was reported as an important procedure in both in-out and out-in (i.e. transforaminal endoscopic spine system (TESSYS)) techniques in the transforaminal endoscopic discectomy (TED), and which was also related to the deterioration of postoperative biomechanical environment and related poor prognosis. -

Priority Health Spine and Joint Code List
Priority Health Joint Services Code List Category CPT® Code CPT® Code Description Joint Services 23000 Removal of subdeltoid calcareous deposits, open Joint Services 23020 Capsular contracture release (eg, Sever type procedure) Joint Services 23120 Claviculectomy; partial Joint Services 23130 Acromioplasty or acromionectomy, partial, with or without coracoacromial ligament release Joint Services 23410 Repair of ruptured musculotendinous cuff (eg, rotator cuff) open; acute Joint Services 23412 Repair of ruptured musculotendinous cuff (eg, rotator cuff) open;chronic Joint Services 23415 Coracoacromial ligament release, with or without acromioplasty Joint Services 23420 Reconstruction of complete shoulder (rotator) cuff avulsion, chronic (includes acromioplasty) Joint Services 23430 Tenodesis of long tendon of biceps Joint Services 23440 Resection or transplantation of long tendon of biceps Joint Services 23450 Capsulorrhaphy, anterior; Putti-Platt procedure or Magnuson type operation Joint Services 23455 Capsulorrhaphy, anterior;with labral repair (eg, Bankart procedure) Joint Services 23460 Capsulorrhaphy, anterior, any type; with bone block Joint Services 23462 Capsulorrhaphy, anterior, any type;with coracoid process transfer Joint Services 23465 Capsulorrhaphy, glenohumeral joint, posterior, with or without bone block Joint Services 23466 Capsulorrhaphy, glenohumeral joint, any type multi-directional instability Joint Services 23470 ARTHROPLASTY, GLENOHUMERAL JOINT; HEMIARTHROPLASTY ARTHROPLASTY, GLENOHUMERAL JOINT; TOTAL SHOULDER [GLENOID -

Musculoskeletal Surgical Procedures Requiring Prior Authorization (Effective 11.1.2020)
Musculoskeletal Surgical Procedures Requiring Prior Authorization (Effective 11.1.2020) Procedure Code Description ACL Repair 27407 Repair, primary, torn ligament and/or capsule, knee; cruciate ACL Repair 27409 Repair, primary, torn ligament and/or capsule, knee; collateral and cruciate ligaments ACL Repair 29888 Arthroscopically aided anterior cruciate ligament repair/augmentation or reconstruction Acromioplasty and Rotator Cuff Repair 23130 Acromioplasty Or Acromionectomy, Partial, With Or Without Coracoacromial Ligament Release Acromioplasty and Rotator Cuff Repair 23410 Repair of ruptured musculotendinous cuff (eg, rotator cuff) open; acute Acromioplasty and Rotator Cuff Repair 23412 Repair of ruptured musculotendinous cuff (eg, rotator cuff) open; chronic Acromioplasty and Rotator Cuff Repair 23415 Coracoacromial Ligament Release, With Or Without Acromioplasty Acromioplasty and Rotator Cuff Repair 23420 Reconstruction of complete shoulder (rotator) cuff avulsion, chronic (includes acromioplasty) Arthroscopy, Shoulder, Surgical; Decompression Of Subacromial Space With Partial Acromioplasty, With Coracoacromial Ligament (Ie, Arch) Release, When Performed (List Separately In Addition Acromioplasty and Rotator Cuff Repair 29826 To Code For Primary Procedure) Acromioplasty and Rotator Cuff Repair 29827 Arthroscopy, shoulder, surgical; with rotator cuff repair Allograft for Spinal Fusion [BMP] 20930 Allograft, morselized, or placement of osteopromotive material, for spine surgery only Ankle Fusion 27870 Arthrodesis, ankle, open Ankle Fusion -

Vertebroplasty and Percutaneous Vertebral Augmentation
Medicare Part C Medical Coverage Policy Vertebroplasty and Percutaneous Vertebral Augmentation Origination Date: December 16, 2002 Vertebroplasty August 20, 2003 Kyphoplasty Review Date: June 17, 2020 Next Review: June, 2022 ***This policy applies to all Blue Medicare HMO, Blue Medicare PPO, Blue Medicare Rx members, and members of any third-party Medicare plans supported by Blue Cross NC through administrative or operational services. *** DESCRIPTION OF PROCEDURE OR SERVICE Vertebroplasty Percutaneous vertebroplasty is a therapeutic, interventional radiologic procedure, which consists of the injection of a biomaterial (usually polymethylmethacrylate- bone cement) under imaging guidance (either fluoroscopy or CT) into a cervical, thoracic or lumbar vertebral body lesion for the relief of pain and the strengthening of bone. Percutaneous Vertebral Augmentation This is also known as balloon-assisted Percutaneous Vertebroplasty or Kyphoplasty. The procedure is similar to percutaneous vertebroplasty in that stabilization of the collapsed vertebra is accomplished by the injection of the same biomaterial into the body of the vertebra. The primary difference is that the fracture is partially reduced with the insertion of an inflatable balloon tamp. Once inflated, the balloon tamp (plug) restores some height to the vertebral body, while creating a cavity that is filled with bone cement. POLICY STATEMENT Coverage will be provided for vertebroplasty or percutaneous vertebral augmentation when it is determined to be medically necessary because the -

MISSED? Metastatic Spinal Cord Compression NA Quraishi, C Esler ∗ BMJ 342 (7805), 1023-1025
PUBLICATIONS (ABSTRACTS EXCLUDED) 2014: Metastatic spinal cord compression as a result of the unknown primary tumour. Quraishi NA, Ramoutar D, Sureshkumar D, Manoharan SR, Spencer A, Arealis G, Edwards KL, Boszczyk BM. Eur Spine J. 2014 Apr 2. Trans-oral approach for the management of a C2 neuroblastoma. Salem KM, Visser J, Quraishi NA. Eur Spine J. 2014 Feb 19. Calcified giant thoracic disc herniations: considerations and treatment strategies. Quraishi NA, Khurana A, Tsegaye MM, Boszczyk BM, Mehdian SM. Eur Spine J. 2014 Apr;23 Surgical treatment of sacral chordoma: prognostic variables for local recurrence and overall survival. Varga PP, Szövérfi Z, Fisher CG, Boriani S, Gokaslan ZL, Dekutoski MB, Chou D, Qurais NA, Reynolds JJ, Luzzati A, Williams R, Fehlings MG, Germscheid NM, Lazary A, Rhines LD. Eur Spine J. 2014 Dec 23. Expert's comment concerning Grand Rounds case entitled: "trans-oral approach for the management of a C2 neuroblastoma. (K. M. I. Salem, J. Visser, and N. A. Quraishi).Choi D. Eur Spine J. 2015 Jan;24(1):177-9. Diagnosis and treatment of a rectal-cutaneous fistula: a rare complication of coccygectomy. Behrbalk E, Uri O, Maxwell-Armstrong C, Quraishi NA. Eur Spine J. 2014 Nov 1. A cohort study to evaluate cardiovascular risk of selective and nonselective cyclooxygenase inhibitors (COX-Is) in arthritic patients attending orthopedic department of a tertiary care hospital. Bhosale UA, Quraishi N, Yegnanarayan R, Devasthale D. Niger Med J. 2014 Sep;55(5):417-22. An evidence-based medicine model for rare and often neglected neoplastic conditions. Fisher CG, Goldschlager T, Boriani S, Varga PP, Rhines LD, Fehlings MG, Luzzati A, Dekutoski MB, Reynolds JJ, Chou D, Berven SH, Williams RP, Quraishi NA, Bettegowda C, Gokaslan ZL. -

A Novel and Convenient Method to Evaluate Bone Cement Distribution Following Percutaneous Vertebral Augmentation
www.nature.com/scientificreports OPEN A novel and convenient method to evaluate bone cement distribution following percutaneous vertebral augmentation Jin Liu1,2,4, Jing Tang3,4, Hao Liu1*, Zuchao Gu2, Yu Zhang2 & Shenghui Yu2 A convenient method to evaluate bone cement distribution following vertebral augmentation is lacking, and therefore so is our understanding of the optimal distribution. To address these questions, we conducted a retrospective study using data from patients with a single-segment vertebral fracture who were treated with vertebral augmentation at our two hospitals. Five evaluation methods based on X-ray flm were compared to determine the best evaluation method and the optimal cement distribution. Of the 263 patients included, 49 (18.63%) experienced re-collapse of treated vertebrae and 119 (45.25%) experienced new fractures during follow-up. A 12-score evaluation method (kappa value = 0.652) showed the largest area under the receiver operating characteristic curve for predicting new fractures (0.591) or re-collapse (0.933). In linear regression with the 12-score method, the bone cement distribution showed a negative correlation with the re-collapse of treated vertebra, but it showed a weak correlation with new fracture. The two prediction curves intersected at a score of 10. We conclude that an X-ray-based method for evaluation of bone cement distribution can be convenient and practical, and it can reliably predict risk of new fracture and re-collapse. The 12-score method showed the strongest predictive power, with a score of 10 suggesting optimal bone cement distribution. Since Galibert and Deramond frst used bone cement to treat aggressive cervical hemangiomas in 19871, this procedure has been verifed by numerous studies to be an efective minimally invasive surgery for the treatment of osteoporotic vertebral compression fractures (OVCFs)2–5.