Chapter 1. Chemical Processes 1.1 Introduction Chemical Processes Usually Have Three Interrelated Steps: 1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1. Define: Unit Operation. Useful Physical Changes Occur in the Chemical Industry Is Known As a Unit Operation
INTREVIEW QUESTIONS 1. Define: Unit operation. Useful Physical changes occur in the chemical industry is known as a Unit Operation. Example: Distillation, Filtration, Drying, Extraction, Gas absorption, Crystallization, etc. 2. Define: Unit process. Useful Chemical changes with or without physical change occur in chemical industry are known as a Unit Process. Example: - Oxidation, Reduction, Alkylation, Sulfonation, Chlorination, etc. 3. Define: Boiling Point and Bubble Point. Boiling Point: -It is a temperature of a liquid at which the vapour pressure of the liquid is equal to atmospheric pressure. Bubble Point: -It is a temperature at which first bubble of vapour is formed. 4. When does liquid boil? When the vapour pressure of the liquid is equal to atmospheric pressure at that time liquid is boil. 5. Define: - Volatile Liquid. It is a tendency of a liquid to vaporize. 6. Acetone and water out of this which is more volatile and why? Acetone is more volatile than water because of boiling point of acetone (56.7 °C) is low compa re to boiling point of water (100° C) 7. What is the Relative Volatility? It is a ratio of concentration of more volatile component in vapour phase to liquid phase is called Relative Volatility 8. What is important of Relative Volatility? For separation of liquid mixture using distillation, Relative volatility should be more than 1. 9. Why is Reflux done in distillation column? and Define Reflux Ratio. For Increasing product purity. Reflux: -It is amount of distillate which is resend to distillation column is known as a reflux. Reflux Ratio: -Reflux ratio is the ratio of the portion of the overhead liquid product from a distillation column that is returned to the upper part of column to the portion of liquid collected as distillate. -

MATERIAL BALANCE NOTES Revision 3 Irven Rinard Department
MATERIAL BALANCE NOTES Revision 3 Irven Rinard Department of Chemical Engineering City College of CUNY and Project ECSEL October 1999 © 1999 Irven Rinard CONTENTS INTRODUCTION 1 A. Types of Material Balance Problems B. Historical Perspective I. CONSERVATION OF MASS 5 A. Control Volumes B. Holdup or Inventory C. Material Balance Basis D. Material Balances II. PROCESSES 13 A. The Concept of a Process B. Basic Processing Functions C. Unit Operations D. Modes of Process Operations III. PROCESS MATERIAL BALANCES 21 A. The Stream Summary B. Equipment Characterization IV. STEADY-STATE PROCESS MODELING 29 A. Linear Input-Output Models B. Rigorous Models V. STEADY-STATE MATERIAL BALANCE CALCULATIONS 33 A. Sequential Modular B. Simultaneous C. Design Specifications D. Optimization E. Ad Hoc Methods VI. RECYCLE STREAMS AND TEAR SETS 37 A. The Node Incidence Matrix B. Enumeration of Tear Sets VII. SOLUTION OF LINEAR MATERIAL BALANCE MODELS 45 A. Use of Linear Equation Solvers B. Reduction to the Tear Set Variables C. Design Specifications i VIII. SEQUENTIAL MODULAR SOLUTION OF NONLINEAR 53 MATERIAL BALANCE MODELS A. Convergence by Direct Iteration B. Convergence Acceleration C. The Method of Wegstein IX. MIXING AND BLENDING PROBLEMS 61 A. Mixing B. Blending X. PLANT DATA ANALYSIS AND RECONCILIATION 67 A. Plant Data B. Data Reconciliation XI. THE ELEMENTS OF DYNAMIC PROCESS MODELING 75 A. Conservation of Mass for Dynamic Systems B. Surge and Mixing Tanks C. Gas Holders XII. PROCESS SIMULATORS 87 A. Steady State B. Dynamic BILIOGRAPHY 89 APPENDICES A. Reaction Stoichiometry 91 B. Evaluation of Equipment Model Parameters 93 C. Complex Equipment Models 96 D. -

Unit Operations for Bioprocess Engineers
Unit Operations for Bioprocess Engineers Chenming (Mike) Zhang Department of Biological Systems Engineering Virginia Polytechnic Institute and State University Blacksburg, VA 24061 Abstract Unit Operations in Biological Systems Engineering was introduced into the curriculum at Virginia Tech in 2000. It is a lecture and laboratory combined course. The lectures and experiments covered in the course had a narrow focus before the author took over in 2002. To broaden the education for students selecting the Bioprocess Engineering option within the curriculum, the author has revised the content of the course to give the students an opportunity to understand that different unit operations can be applied in various industries, namely food, biochemical, and biotechnology. The experiments have been decreased from 14 to 8 so the students could have a better grasp of the theories and applications. Students’ responses showed that it is important to design the experiments in a way to stimulate their desire to learn and perform. The author found that it is possible to give the students a better view of the various unit operations in bioprocess engineering. Page 9.1342.1 Page 1 Introduction As defined by Shuler and Kargi (2002), “Bioprocess engineers are engineers working to apply the principles of various disciplines, such as chemical, mechanical, electrical, and industrial, to processes based on using living cells or subcomponents of such cells.” In other words, bioprocess engineers are engineers who process biological materials to produce useful goods for society. Without question, bioprocess engineering is a broad-based engineering discipline. As educators, it is our job to broaden the view of the students, so they can take advantage of numerous, diverse job opportunities presented to them when they finish their BS degree. -

Yntbietic Fnei
23c$b/J jflt yntbietic fnei OIL SHALE 0 COAL 0 OIL SANDS VOLUME 24 — NUMBER 3 — SEPTEMBER 1987 QUARTERLY Toll Eril Reposito,y ,.r;.'.ur Lakas Library csdo School of Mcs © THE PACE CONSULTANTS INC. 0 Reg. U.S. Pal. OFF. Pace Synthetic Fuels Report Is published by The Pace Consultants Inc., as a multi-client service and is intended for the sole use of the clients or organizations affiliated with clients by virtue of a relationship equivalent to 51 percent or greater ownership. Pace Synthetic Fuels Report is protected by the copyright laws of the United States; reproduction of any part of the publication requires the express permission of The Pace Con- sultants Inc. The Pace Consultants Inc., has provided energy consulting and engineering services since 1955. The company's experience includes resource evalua- tion, process development and design, systems planning, marketing studies, licenser comparisons, environmental planning, and economic analysis. The Synthetic Fuels Analysis group prepares a variety of periodic and other reports analyzing developments in the energy field. THE PACE CONSULTANTS INC. SYNTHETIC FUELS ANALYSIS MANAGING EDITOR Jerry E. Sinor Pt Office Box 649 Niwot, Colorado 80544 (303) 652-2632 BUSINESS MANAGER Ronald L. Gist Post Office Box 53473 Houston, Texas 77052 (713) 669-8800 Telex: 77-4350 CONTENTS HIGHLIGHTS A-i I. GENERAL CORPORATIONS Gill Cuts Coal Gasification Programs 1-i API Data Shows Drop in Oil Production 1-4 GOVERNMENT Petroleum Research Institute Proposed i-S FERC Kills Incremental Gas Pricing 1-5 California to Fund Advanced Energy Technologies i_s ERAB Studying Energy Competitiveness 1-6 ENERGY POLICY AND FORECASTS U.S. -

Making Downstream Processing Continuous and Robust a Virtual Roundtable S
BIOPROCESS TECHNICAL Making Downstream Processing Continuous and Robust A Virtual Roundtable S. Anne Montgomery, Cheryl Scott, and Peter Satzer, with Margit Holzer, Miriam Monge, Ralph Daumke, and Alexander Faude urrent biomanufacturing is driven to pursue continuous processing for cost reduction and increased productivity, Cespecially for monoclonal antibody (MAb) production and manufacturing. Although many technologies are now available and have been implemented in biodevelopment, implementation for large-scale production is still in its infancy. In a lively roundtable discussion at the BPI West conference in Santa Clara, CA (11 March 2019), participants touched on a number of important issues still to be resolved and technologies that are still in need of implementation at large scale. Below, moderator Peter Satzer (senior scientist RENTSCHLER BIOPHARMA (WWW.RENTSCHLER-BIOPHARMA.COM) with the Austrian Center of Industrial Biotechnology, Vienna) summarizes key points raised in that session. Based on Requirements for integration without the need for hold his highlights, BPI asked a number of Continuous Downstream tanks between unit operations requires industry representatives to comment on further exploration. those points further, and their Applications Chromatography Operations: Some responses follow. by Peter Satzer unit operations such as flow-through chromatography and single-pass, Minimizing Buffer and Hold Tanks: One tangential-flow filtration (SPTFF) can be critical parameter is integration of unit integrated easily into a continuous Product Focus: Biologics operations on manufacturing shop downstream process scheme at any floors using minimum numbers of point because they offer constant inflow Process Focus: Downstream buffer tanks and hold tanks. The benefit and outflow of material. Such operations processing of continuous manufacturing can be can be called truly or fully continuous. -

Green Petroleum Refining - Mathematical Models for Optimizing Petroleum Refining Under Emission Constraints
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by University of Waterloo's Institutional Repository Green Petroleum Refining - Mathematical Models for Optimizing Petroleum Refining Under Emission Constraints by Yusuf Ali Yusuf A thesis presented to the University of Waterloo in fulfillment of the thesis requirement for the degree of Master of Applied Science in Chemical Engineering Waterloo, Ontario, Canada, 2013 © Yusuf Ali Yusuf 2013 I hereby declare that I am the sole author of this thesis. This is a true copy of the thesis, including any required final revisions, as accepted by my examiners. I understand that my thesis may be made electronically available to the public. ii Abstract Petroleum refining processes provide the daily requirements of energy for the global market. Each refining process produces wastes that have the capacity to harm the environment if not properly disposed of. The treatment of refinery waste is one of the most complex issues faced by refinery managers. Also, the hazardous nature of these wastes makes them rather costly to dispose of for the refineries. In this thesis, system analysis tools are used to design a program that allows for the selection of the optimal control, minimization and treating options for petroleum refinery waste streams. The performance of the developed model is demonstrated via a case study. Optimal mitigation alternatives to meet the emission reduction targets were studied by evaluating their relative impact on the profitable operation of the given facility. It was found that the optimal mitigation steps was to reduce emission precursors by conducting feed switches at the refinery. -

Ac 2011-1640: Unit Operations Lab Bazaar
AC 2011-1640: UNIT OPERATIONS LAB BAZAAR Michael E Prudich, Ohio University Mike Prudich is a professor in the Department of Chemical and Biomolecular Engineering at Ohio Uni- versity were he has been for 27 years. Prior to joining the faculty at Ohio University, he was a senior research engineering at Gulf Research and Development Company in Pittsburgh, PA primarily working in the area of synthetic fuels. Daina Briedis, Michigan State University DAINA BRIEDIS is a faculty member in the Department of Chemical Engineering and Materials Science at Michigan State University. Dr. Briedis has been involved in several areas of education research includ- ing student retention, curriculum redesign, and the use of technology in the classroom. She is a co-PI on two NSF grants in the areas of integration of computation in engineering curricula and in developing comprehensive strategies to retain early engineering students. She is active nationally and internationally in engineering accreditation and is a Fellow of ABET. Robert Y. Ofoli, Michigan State University ROBERT Y. OFOLI is an associate professor in the Department of Chemical Engineering and Materi- als Science at Michigan State University. He has had a long interest in teaching innovations, and has used a variety of active learning protocols in his courses. His research interests include biosensors for biomedical applications, optical and electrochemical characterization of active nanostructured interfaces, nanocatalytic conversion of biorenewables to commodity chemicals and fuels, and nanoscale production of hydrogen on demand. Robert B. Barat, New Jersey Institute of Technology Robert Barat is a Professor of Chemical Engineering at NJIT, where he has been a faculty member for over 20 years. -

Marko Leino Process Simulation Unit Operation Models – Review of Open
MARKO LEINO PROCESS SIMULATION UNIT OPERATION MODELS – REVIEW OF OPEN AND HSC CHEMISTRY I/O INTERFACES Master of Science Thesis Examiner: Professor Hannu Jaakkola Examiner and topic approved by the Faculty Council of the Faculty of Business and Built Environment on 6 April 2016 i ABSTRACT MARKO LEINO: Process Simulation Unit Operation Models – Review of Open and HSC Chemistry I/O Interfaces Tampere University of Technology Master of Science Thesis, 76 pages, 29 Appendix pages April 2016 Master’s Degree Programme in Information Technology Major: Software Engineering Examiner: Professor Hannu Jaakkola Keywords: CAPE-OPEN, HSC Chemistry Sim, unit operation, process simulation Chemical process modelling and simulation can be used as a design tool in the development of chemical plants, and is utilized as a means to evaluate different design options. The CAPE- OPEN interface standards were developed to allow the deployment and utilization of process modelling components in any compliant process modelling environment. This thesis examines the possibilities provided by the CAPE-OPEN interfaces and the .NET framework to develop compliant, cross-platform process modelling components, particularly unit operations. From the software engineering point of view, a unit operation is a represen- tation of physical equipment, and contains the mathematical model of its functionality. The study indicates that the differences between the CAPE-OPEN standards and Outotec HSC Chemistry Sim are negligible at the conceptual level. On the other hand, at the implementa- tion level, the differences are quite considerable. Regardless of the simulation application be- ing used, the modelling of unit operations requires interdisciplinary skills, and creating tools and methods to ease the development of such models is well justified. -

Chemical Process Simulation Using Openmodelica Priyam Nayak, Pravin Dalve, Rahul Anandi Sai, Rahul Jain, Kannan M
Chemical Process Simulation Using OpenModelica Priyam Nayak, Pravin Dalve, Rahul Anandi Sai, Rahul Jain, Kannan M. Moudgalya, P. R. Naren, Peter Fritzson and Daniel Wagner The self-archived postprint version of this journal article is available at Linköping University Institutional Repository (DiVA): http://urn.kb.se/resolve?urn=urn:nbn:se:liu:diva-159151 N.B.: When citing this work, cite the original publication. Nayak, P., Dalve, P., Sai, R. A., Jain, R., Moudgalya, K. M., Naren, P. R., Fritzson, P., Wagner, D., (2019), Chemical Process Simulation Using OpenModelica, Industrial & Engineering Chemistry Research, 58(26), 11164-11174. https://doi.org/10.1021/acs.iecr.9b00104 Original publication available at: https://doi.org/10.1021/acs.iecr.9b00104 Copyright: American Chemical Society http://pubs.acs.org/ Article Cite This: Ind. Eng. Chem. Res. XXXX, XXX, XXX−XXX pubs.acs.org/IECR Chemical Process Simulation Using OpenModelica † † † † † Priyam Nayak, Pravin Dalve, Rahul Anandi Sai, Rahul Jain, Kannan M. Moudgalya,*, ‡ ¶ § P. R. Naren, Peter Fritzson, and Daniel Wagner † Department of Chemical Engineering, IIT Bombay, Mumbai 400 076, India ‡ School of Chemical & Biotechnology, SASTRA Deemed University, Thanjavur 613 401, India ¶ Linkoping University, 581 83 Linköping, Sweden § Independent Researcher, Manaus 69053-020, Brazil ABSTRACT: The equation-oriented general-purpose simulator OpenModelica provides a convenient, extendible modeling environ- ment, with capabilities such as an easy switch from steady-state to dynamic simulations. This work reports the creation of a library of steady-state models of unit operations using OpenModelica. The use of this library is demonstrated through a few representative flow- sheets, and the results are compared with the steady-state simulators Aspen Plus and DWSIM. -

Transport Phenomena Vs Unit Operations Approach
Chapter 2 Transport Phenomena vs Unit Operations Approach The history of Unit Operations is interesting. As indicated in the previous chapter, chemical engineering courses were originally based on the study of unit processes and/or industrial technologies. However, it soon became apparent that the changes produced in equipment from different industries were similar in nature, i.e., there was a commonality in the mass transfer operations in the petroleum industry as with the utility industry. These similar operations became known as Unit Operations. This approach to chemical engineering was promulgated in the Little report discussed earlier, and has, with varying degrees and emphasis, dominated the profession to this day. The Unit Operations approach was adopted by the profession soon after its inception. During the 130 years (since 1880) that the profession has been in existence as a branch of engineering, society’s needs have changed tremendously and so has chemical engineering. The teaching of Unit Operations at the undergraduate level has remained rela- tively unchanged since the publication of several early- to mid-1900 texts. However, by the middle of the 20th century, there was a slow movement from the unit operation concept to a more theoretical treatment called transport phenomena or, more simply, engineering science. The focal point of this science is the rigorous mathematical description of all physical rate processes in terms of mass, heat, or momentum crossing phase boundaries. This approach took hold of the education/curriculum of the profession with the publication of the first edition of the Bird et al. book.(1) Some, including both authors of this text, feel that this concept set the profession back several decades since graduating chemical engineers, in terms of training, were more applied physicists than traditional chemical engineers. -
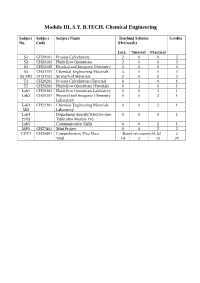
Module III, S.Y. B.TECH. Chemical Engineering
Module III, S.Y. B.TECH. Chemical Engineering Subject Subject Subject Name Teaching Scheme Credits No. Code (Hrs/week) Lect. Tutorial Practical S1 CH20101 Process Calculations 3 0 0 3 S2 CH20103 Fluid-flow Operations 3 0 0 3 S3 CH20105 Physical and Inorganic Chemistry 3 0 0 3 S4 CH21101 Chemical Engineering Materials 3 0 0 3 S5 MD CH21103 Strength of Materials 2 0 0 2 T1 CH20201 Process Calculations (Tutorial) 0 1 0 1 T2 CH20203 Fluid-flow Operations (Tutorial) 0 1 0 1 Lab1 CH20303 Fluid-flow Operations Laboratory 0 0 2 1 Lab2 CH20307 Physical and Inorganic Chemistry 0 0 2 1 Laboratory Lab3 CH21301 Chemical Engineering Materials 0 0 2 1 MD Laboratory Lab4 Department Specific Elective (see 0 0 2 1 (SD) Table after Module IV) Lab5 Communication Skills 0 0 2 1 MP3 CH27401 Mini Project 0 0 2 2 CVV1 CH20401 Comprehensive Viva Voce Based on courses S1,S2 2 Total 14 2 12 25 Issue 05: Rev No. 1: Dt. 30/03/15 FF No: 654A CH 20101:: PROCESS CALCULATIONS Credits: 03 Teaching Scheme: 3 Hours / Week Unit I: Basic Chemical Calculations (8 Hrs) A. Dimensions and Units, chemical calculations including mole, equivalent weight, solids, liquids, solutions and their properties, properties of gases. B. Significance Unit conversions of mass, energy and pressure Unit II: Material Balances Without Chemical Reactions (8 Hrs) A. Process flow sheet, Concept, Material balance calculations, recycling and bypassing operations, material balance of unsteady state processes. B. Material balance of unit operations such as distillation, crystallization Unit III: Material Balances Involving Chemical Reactions (8 Hrs) A. -

When Chemical Reactors Were Admitted and Earlier Roots of Chemical Engineering
When Chemical Reactors Were Admitted And Earlier Roots of Chemical Engineering 9 Biographical sketch of L. E. ‘Skip’ Scriven L. E. 'Skip' Scriven is Regents' Professor and holder of the L E Scriven Chair of Chemical Engineering & Materials Science at the University of Minnesota. He is a Fellow of the Minnesota Supercomputer Institute, founded the Coating Process Fundamentals Program, and now co-leads it with Professor Lorraine F. Francis. He is distinguished for pioneering researches in several areas of fluid mechanics, interfacial phenomena, porous media and surfactant technologies, and the recently emerged field of coating science and engineering. He promoted close interactions with industry by showing how good theory, incisive experimental techniques, and modern computer-aided mathematics can be combined to solve industrial processing problems. He graduated from the University of California, Berkeley, received a Ph.D. from the University of Delaware, and was a research engineer with Shell Development Company for four years before joining the University of Minnesota. He received the AIChE Allan P. Colburn Award four decades ago, the William H. Walker Award two decades ago, the Tallmadge Award in 1992, and the Founders Award in 1997. He has also been honored by the University of Minnesota and the American Society for Engineering Education for outstanding teaching. He has co-advised or advised many undergraduate, graduate and postdoctoral research students, including over 100 Ph.D.’s. Elected to the National Academy of Engineering in 1978, he has served on several U.S. national committees setting priorities for chemical engineering and materials science research. In 1990-92 he co-chaired the National Research Council's Board on Chemical Sciences and Technology, and in 1994-97 he served on the governing Commission on Physical Sciences, Mathematics, and Applications.