Assessment of Fluoride Contaminations in Groundwater of Hard Rock
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
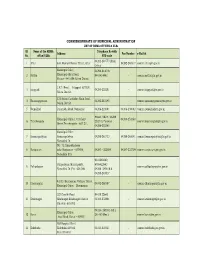
LIST of RDMA OFFICES & Ulbs
COMMISSIONERATE OF MUNICIPAL ADMINISTRATION LIST OF RDMA OFFICES & ULBs Sl. Name of the RDMA Telephone No with Address Fax Number e-Mail id No. office/ULBs STD code 04282-240773 (Main 1 Attur 634, Ranipet Bazaar Street, Attur 04282-240317 [email protected] Office) Municipal Office, 04298-244018, 2 Mettur Municipal office Road, 9443414443 - [email protected] Mettur – 641 604,Salem District J.K.P. Road, Idappadi-637101, 3 Idappadi 04283-222201 - [email protected] Salem District. 121 Salem Cuddalore Main Road, 4 Narasingapuram 04282-281495 - [email protected] Salem District 5 Namakkal Paramathi Road, Namakkal 04286-221001 04286-230002 [email protected] Phone: Office : 04288 Municipal Office,1, Cutchery 04288-252360 6 Tiruchengode 252310, Personal : [email protected] Street,Tiruchengode - 637 211, 04288-252360 Municipal Office 7 Komarapalayam Komarapalayam 04288-261733 04288-261041 [email protected] Namakkal Dt. NO. 72, Bharathidasan 8 Rasipuram salai,Rasipuram - 637408, 04287 – 222859 04287-222709 [email protected] Namakkal (Dt) 9444289849, Pallipalayam Municipality, 9789422842 9 Pallipalayam - [email protected] Namakkal Dt.,Pin : 638 006 04288- 240816 & 04288-240817 43/13, Kandasamy Vathiyar Street, 10 Dharmapuri 04342-260387 - [email protected] Municipal Office, Dharmapuri 327 Gandhi Road 94439 22640 11 Krishnagiri Krishnagiri.Krishnagiri District. 04343-232664 - [email protected] Pin code -635 001 04324- 260341 (Off.) Municipal Office, 12 Karur 261480 (Res.) - [email protected] Azad Road, Karur – 639001 Old Hospital Street 13 Kulithalai Kulithalai-639104 04323-222321 - [email protected] Karur District 14 Theni-Allinagaram Theni-Allinagaram Municipal 04546-252470 -- [email protected] Office, Nehruji Road, Theni Allinagaram - 625 531. -

District Survey Report of Madurai District
Content 1.0 Preamble ................................................................................................................. 1 2.0 Introduction .............................................................................................................. 1 2.1 Location ............................................................................................................ 2 3.0 Overview of Mining Activity In The District .............................................................. 3 4.0 List of Mining Leases details ................................................................................... 5 5.0 Details of the Royalty or Revenue received in last Three Years ............................ 36 6.0 Details of Production of Sand or Bajri Or Minor Minerals In Last Three Years ..... 36 7.0 Process of deposition of Sediments In The River of The District ........................... 36 8.0 General Profile of Maduari District ....................................................................... 27 8.1 History ............................................................................................................. 28 8.2 Geography ....................................................................................................... 28 8.3 Taluk ................................................................................................................ 28 8.2 Blocks .............................................................................................................. 29 9.0 Land Utilization Pattern In The -
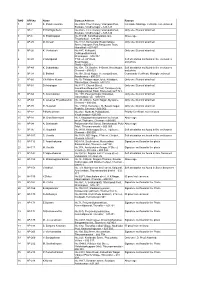
SNO APP.No Name Contact Address Reason 1 AP-1 K
SNO APP.No Name Contact Address Reason 1 AP-1 K. Pandeeswaran No.2/545, Then Colony, Vilampatti Post, Intercaste Marriage certificate not enclosed Sivakasi, Virudhunagar – 626 124 2 AP-2 P. Karthigai Selvi No.2/545, Then Colony, Vilampatti Post, Only one ID proof attached. Sivakasi, Virudhunagar – 626 124 3 AP-8 N. Esakkiappan No.37/45E, Nandhagopalapuram, Above age Thoothukudi – 628 002. 4 AP-25 M. Dinesh No.4/133, Kothamalai Road,Vadaku Only one ID proof attached. Street,Vadugam Post,Rasipuram Taluk, Namakkal – 637 407. 5 AP-26 K. Venkatesh No.4/47, Kettupatti, Only one ID proof attached. Dokkupodhanahalli, Dharmapuri – 636 807. 6 AP-28 P. Manipandi 1stStreet, 24thWard, Self attestation not found in the enclosures Sivaji Nagar, and photo Theni – 625 531. 7 AP-49 K. Sobanbabu No.10/4, T.K.Garden, 3rdStreet, Korukkupet, Self attestation not found in the enclosures Chennai – 600 021. and photo 8 AP-58 S. Barkavi No.168, Sivaji Nagar, Veerampattinam, Community Certificate Wrongly enclosed Pondicherry – 605 007. 9 AP-60 V.A.Kishor Kumar No.19, Thilagar nagar, Ist st, Kaladipet, Only one ID proof attached. Thiruvottiyur, Chennai -600 019 10 AP-61 D.Anbalagan No.8/171, Church Street, Only one ID proof attached. Komathimuthupuram Post, Panaiyoor(via) Changarankovil Taluk, Tirunelveli, 627 761. 11 AP-64 S. Arun kannan No. 15D, Poonga Nagar, Kaladipet, Only one ID proof attached. Thiruvottiyur, Ch – 600 019 12 AP-69 K. Lavanya Priyadharshini No, 35, A Block, Nochi Nagar, Mylapore, Only one ID proof attached. Chennai – 600 004 13 AP-70 G. -

Khadi Institution Profile Khadi and Village Industries Comission
KHADI AND VILLAGE INDUSTRIES COMISSION KHADI INSTITUTION PROFILE Office Name : SO CHENNAI TAMIL NADU Institution Code : 99027 Institution Name : TAMIL NADU KHADI & V. I. BOARD, Address: : KURALAGAM ANNEXE, 5TH FLOOR, Post : Chennai City/Village : Chennai Pincode : 600104 State : TAMIL NADU District : CHENNAI Aided by : KVIB District : A+ Contact Person Name Email ID Mobile No. Chairman : L.K.TIRUPATHY [email protected] 9566012097 Secretary : Nodal Officer : Registration Detail Registration Date Registration No. Registration Type 12-11-2020 99027 SOC Khadi Certificate No. TND/1254 Date : 31-MAR_2021 Khadi Mark No. 0068 Khadi Mark Dt. 01-Oct-2019 Sales Outlet Details Type Name Address City Pincode Sales Outlet KHADI KRAFT SHANMUGAR SALAI MADRAS 600045 ROAD ,TAMBARAM Sales Outlet KHADI KRAFT NORTH PARK AMBATTUR 600052 STREET , AMBATTUR Sales Outlet GRAMSHILPA KURUUNJIPADI, LUDDALORE 607302 NO.11, BUS STAND Sales Outlet KHADI KRAFT 99/ ERODE MAIN TIRUCHCHIRAPPAL 639104 ROAD, LI Sales Outlet KHADI KRAFT THOTHIYAM, THOTHIYAM 621215 TIRUCHCHIRAPPAL LI Sales Outlet KHADI KRAFT NO-294, K H ROAD AYANAVARM 600023 Sales Outlet KHADI KRAFT NO-173 G A ROAD, CHENNAI 600021 Sales Outlet KHADI KRAFT A D2 BLUE STAR, ANNANAGAR 600040 ANNA NAGAR Sales Outlet KHADI KRAFT 172, ANNASALAI ANNA SALAI 600002 Sales Outlet KHADI KRAFT THIRAVATTIYAR, CHENNAI 600019 Sales Outlet KHADI KRAFT NO-9 ESPLANADE, PARRYS 600001 KURALAGAM Sales Outlet DEPARTMENTAL UNIT OF 26,27 BROTHESTER NAZARATH 600018 KVIB STREET Sales Outlet KHADI KRAFT NO-9 ESPLANADE, PARRYS 600001 KURALAGAM 01 October 2021 Page 1 of 7 Sales Outlet KHADI KRAFT MYLAPORE CHENNAI 600028 ROAD,CHENNAI Sales Outlet DEPARTMENTAL UNIT OF 26,27 BROTHESTER NAZARATH 628617 KVIB STREET Sales Outlet KHADI KRAFT PALANI MAIN ROAD KHADI KRAFT UDU, 642204 , UDUMALPET PALANI M Sales Outlet KHADI KRAFT KUMAR NAGAR , THATTI POLYAM 676103 TIRYAR Sales Outlet KHADI KRAFT PENNAGARAM DHARMAPURI 63670 ROAD , Sales Outlet KHADI KRAFT 7/95, M.G. -

Government of Tamilnadu Tamilnadu Irrigated
GOVERNMENT OF TAMILNADU TAMILNADU IRRIGATED AGRICULTURE MODERNISATION AND WATER BODIES RESTORATION AND MANAGEMENT PROJECT (TN – IAMWARM) PROCUREMENT OF WORKS BIDDING DOCUMENT 02/IAMWARM/WRD/TKR/Addl.Schemes/Works/2013-2014 REHABILITATION OF LEFTOUT LINING OF BRANCH CANAL IV & V OF THIRUMANGALAM MAIN CANAL IN THERKKAR SUB BASIN IN USILAMPATTI TALUK OF MADURAI DISTRICT TAMIL NADU – SOUTH INDIA. GOVERNMENT OF TAMILNADU TAMILNADU IRRIGATED AGRICULTURE MODERNISATION AND WATER BODIES RESTORATION AND MANAGEMENT PROJECT (TN – IAMWARM) PROCUREMENT OF WORKS BIDDING DOCUMENT 02/IAMWARM/WRD/TKR/Addl.Schemes/Works/2013-2014 REHABILITATION OF LEFTOUT LINING OF BRANCH CANAL IV & V OF THIRUMANGALAM MAIN CANAL IN THERKKAR SUB BASIN IN USILAMPATTI TALUK OF MADURAI DISTRICT TAMIL NADU - SOUTH INDIA Issue to …………………………………………………………………………………….. …………………………………………………………………………………….. ……………………………………………………………………………………… Superintending Engineer, PWD, Periyar Vaigai Basin Circle, WRD, Madurai. GOVERNMENT OF TAMIL NADU TAMIL NADU IRRIGATED AGRICULTURE MODERNISATION AND WATER BODIES RESTORATION AND MANAGEMENT PROJECT (TN IAMWARM) AGREEMENT NO LOAN-4846-IN AND CREDIT – 4255 NATIONAL COMPETITIVE BIDDING (CIVIL WORKS) NAME OF WORK : REHABILITATION OF LEFTOUT LINING OF BRANCH CANAL IV & V OF THIRUMANGALAM MAIN CANAL IN THERKKAR SUB BASIN IN USILAMPATTI TALUK OF MADURAI DISTRICT PERIOD OF SALE OF : FROM 29.11.2013 TO 30.12.2013 BIDDING DOCUMENT TIME AND DATE OF : DATE 19.12.2013 - 11.00 HOURS IST PRE-BID CONFERENCE LAST DATE AND TIME FOR : DATE 31.12.2013 TIME 15.00 HOURS IST RECEIPT OF BIDS TIME AND DATE OF OPENING : DATE 31.12.2013 TIME 15.30 HOURS IST OF BIDS PLACE OF OPENING OF BIDS : Office of the Superintending Engineer, PWD, Periyar Vaigai Basin Circle, Madurai 625 001. -

Tamil Nadu Government Gazette
© [Regd. No. TN/CCN/467/2012-14. GOVERNMENT OF TAMIL NADU [R. Dis. No. 197/2009. 2013 [Price: Rs. 27.20 Paise. TAMIL NADU GOVERNMENT GAZETTE PUBLISHED BY AUTHORITY No. 10] CHENNAI, WEDNESDAY, MARCH 13, 2013 Maasi 29, Nandhana, Thiruvalluvar Aandu–2044 Part VI—Section 4 Advertisements by private individuals and private institutions CONTENTS PRIVATE ADVERTISEMENTS Pages Change of Names .. 553-619 Notice .. 620 NOTICE NO LEGAL RESPONSIBILITY IS ACCEPTED FOR THE PUBLICATION OF ADVERTISEMENTS REGARDING CHANGE OF NAME IN THE TAMIL NADU GOVERNMENT GAZETTE. PERSONS NOTIFYING THE CHANGES WILL REMAIN SOLELY RESPONSIBLE FOR THE LEGAL CONSEQUENCES AND ALSO FOR ANY OTHER MISREPRESENTATION, ETC. (By Order) Director of Stationery and Printing. CHANGE OF NAMES 8416. I, Barakathu Nisha, wife of Thiru Syed Ahamed 8419. My son, P. Jayakodi, born on 10th October 2010 Kabeer, born on 1st April 1966 (native district: (native district: Virudhunagar), residing at Old No. 8-23, New Ramanathapuram), residing at Old No. 4-66, New No. 4/192, No. 8-99, West Street, Veeranapuram, Kalingapatti, Chittarkottai Post, Ramanathapuram-623 513, shall henceforth Sankarankoil Taluk, Tirunelveli-627 753, shall henceforth be be known as BARAKATH NEESHA. known as V.P. RAJA. BARAKATHU NISHA. M. PERUMALSAMY. Ramanathapuram, 4th March 2013. Tirunelveli, 4th March 2013. (Father.) 8420. I, Sarika Kantilal Rathod, wife of Thiru Shripal, born 8417. I, S Rasia Begam, wife of Thiru M. Sulthan, born on on 3rd February 1977 (native district: Chennai), residing at 2nd June 1973 (native district: Dindigul), residing at Old No. 140-13, Periyasamy Road, R.S. Puram Post, Coimbatore- No. 4/115-E, New No. -

Prefix Gpfno Name 06/96 04/98 09/13 07/03 02/12 04/93 01/15 08/10 11/91 11/09 03/99 09/91 07/91 09/92 07/91 10/91 03/97 05/13 01
Subscribers with one or two missing credits upto February 2015 18-JAN-21 05:32 PM (Press Ctrl+F and type your gpf number to identify your record in this list) Send recovery amount of subs/refund and month of encashment for clearance of missing credit(s) PREFIX GPFNO NAME DRAWING AND DISBURSING OFFICER MISSING MONTHS AGRI 23136 SENTHIL M SUPDT ENGR AE)LAND DEVELOPMENT O/O THE CHIEF ENGR(AE) CH 35 06/96 , 27131 JOHN DURAIRAJ S ADMN OFFICER O\O THE JOINT DIR OF AGRICULTURE TIRUVALLUR 04/98 , 09/13 , 27365 BALAN S DY DIR OF AGRI WATER MGT TRAINING CENTRE VINAYAGAPURAM MDU 07/03 , 28080 PARTHIBAN K ASST DIR OF AGRI SARKARSAMAKULAM COIMBATORE DIST 02/12 , 28216 AGALYASAMUNDESWARI C JOINT DIRECTOR OF AGRI COIMBATORE 04/93 , 28244 SELVARAJU S ASST DIR OF AGRI PAVINJUR KANCHEEPURAM DIST 01/15 , 08/10 , 28620 VINAYAGAMOORTHY K ASST DIR OF AGRI RAJAPALAYAM 11/91 , 28651 MOHAN DOSS M ASST EXE ENGR (AE) TANJORE 11/09 , 28748 RAVICHANDAR A K ASST EXE ENGR (AE) KUMBAKONAM 03/99 , 09/91 , 28820 MANI R ASST DIR OF AGRI MELBHUVANAGIRI CUDDALORE DIST 07/91 , 09/92 , 28832 BALASUBRAMANIAN T ASST DIR OF AGRI VALANGAIMAN TIRUVARUR 07/91 , 10/91 , 28957 EMMANUEL RAJAA D DY DIR OF AGRI PA TO COLLECTOR (AGRI) COIMBATORE 03/97 , 05/13 , 28960 SAMPATH KUMAR V ADMN OFFICER DIR OF AGRL CHEPAUK CHENNAI - 5 01/95 , 11/91 , 29126 CHANDRANAGAR T DY DIR OF AGRI (AGRI BUSINESS) VIRUDHUNAGAR 11/91 , 29258 RAMASWAMY K ASST EXE ENGR (AE) KRISHNAGIRI 05/93 , 29317 DEVARAJ I ASST EXE ENGR AGRL ENGINEERAING DEPT UDUMALPET TIRUPPUR DIST 09/00 , 29447 KUMARASAMI D DY DIR OF AGRI -

Madurai District 12 5
Page Chapter Content No. 1. Introduction 1 2. Overview of Mining Activity in the District 4 3. General Profile of the District 6 4. Geology of Madurai District 12 5. Drainage of Irrigation pattern 17 Land Utilisation Pattern in the District: Forest, Agricultural, 6. 18 Horticultural, Mining etc., 7. Surface Water and Ground Water scenario of the District 20 8. Climate and Rainfall of the District 21 9. Details of Mining Leases in the District 23 10. Details of Royalty or Revenue received in last three years 28 11. Details of Production of Minor Mineral in last three years 29 12. Mineral Map of the District 30 List of Letter of Intent (LOI) Holder in the District along with 13. 31 its validity 14. Total Mineral Reserve available in the district 32 15. Quality/Grade of Mineral available in the district 33 16. Use of Mineral 34 17. Demand and supply of the Mineral in the last three years 34 18. Mining Leases marked on the map of the district 35 Details of the area of where there is a cluster of the mining 19. 36 leases 20. Details of Eco-sensitive area 36 21. Impact on the environment due to Mining activity 37 Remedial measures to mitigate the impact of mining on the 22. 38 environment 23. Reclamation of the mined out area 39 24. Risk assessment & Disaster Management Plan 39 25. Details of Occupational health issue in the District 41 Plantation and Green belt development in respect of leases 26. 41 already granted in the district 27. Any other information 42 LIST OF PLATES Plate 1. -
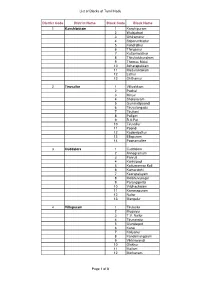
List of Blocks of Tamil Nadu District Code District Name Block Code
List of Blocks of Tamil Nadu District Code District Name Block Code Block Name 1 Kanchipuram 1 Kanchipuram 2 Walajabad 3 Uthiramerur 4 Sriperumbudur 5 Kundrathur 6 Thiruporur 7 Kattankolathur 8 Thirukalukundram 9 Thomas Malai 10 Acharapakkam 11 Madurantakam 12 Lathur 13 Chithamur 2 Tiruvallur 1 Villivakkam 2 Puzhal 3 Minjur 4 Sholavaram 5 Gummidipoondi 6 Tiruvalangadu 7 Tiruttani 8 Pallipet 9 R.K.Pet 10 Tiruvallur 11 Poondi 12 Kadambathur 13 Ellapuram 14 Poonamallee 3 Cuddalore 1 Cuddalore 2 Annagramam 3 Panruti 4 Kurinjipadi 5 Kattumannar Koil 6 Kumaratchi 7 Keerapalayam 8 Melbhuvanagiri 9 Parangipettai 10 Vridhachalam 11 Kammapuram 12 Nallur 13 Mangalur 4 Villupuram 1 Tirukoilur 2 Mugaiyur 3 T.V. Nallur 4 Tirunavalur 5 Ulundurpet 6 Kanai 7 Koliyanur 8 Kandamangalam 9 Vikkiravandi 10 Olakkur 11 Mailam 12 Merkanam Page 1 of 8 List of Blocks of Tamil Nadu District Code District Name Block Code Block Name 13 Vanur 14 Gingee 15 Vallam 16 Melmalayanur 17 Kallakurichi 18 Chinnasalem 19 Rishivandiyam 20 Sankarapuram 21 Thiyagadurgam 22 Kalrayan Hills 5 Vellore 1 Vellore 2 Kaniyambadi 3 Anaicut 4 Madhanur 5 Katpadi 6 K.V. Kuppam 7 Gudiyatham 8 Pernambet 9 Walajah 10 Sholinghur 11 Arakonam 12 Nemili 13 Kaveripakkam 14 Arcot 15 Thimiri 16 Thirupathur 17 Jolarpet 18 Kandhili 19 Natrampalli 20 Alangayam 6 Tiruvannamalai 1 Tiruvannamalai 2 Kilpennathur 3 Thurinjapuram 4 Polur 5 Kalasapakkam 6 Chetpet 7 Chengam 8 Pudupalayam 9 Thandrampet 10 Jawadumalai 11 Cheyyar 12 Anakkavoor 13 Vembakkam 14 Vandavasi 15 Thellar 16 Peranamallur 17 Arni 18 West Arni 7 Salem 1 Salem 2 Veerapandy 3 Panamarathupatti 4 Ayothiyapattinam Page 2 of 8 List of Blocks of Tamil Nadu District Code District Name Block Code Block Name 5 Valapady 6 Yercaud 7 P.N.Palayam 8 Attur 9 Gangavalli 10 Thalaivasal 11 Kolathur 12 Nangavalli 13 Mecheri 14 Omalur 15 Tharamangalam 16 Kadayampatti 17 Sankari 18 Idappady 19 Konganapuram 20 Mac. -
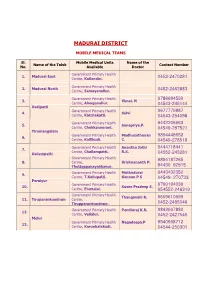
Madurai District
MADURAI DISTRICT MOBILE MEDICAL TEAMS Sl. Mobile Medical Units Name of the Name of the Taluk Contact Number No. Available Doctor Government Primary Health 1. Madurai East 0452-2470281 Centre, Kallandiri. Government Primary Health 2. Madurai North 0452-2463883 Centre, Samayanallur. Government Primary Health 9786684599 3. Vimal. N Centre, Alanganallur. 04543-245144 Vadipatti Government Primary Health 9677779887 4. Selvi Centre, Katchakatti. 04543-254098 Government Primary Health 9442206963 5. Sonapriya.P. Centre, Chekkanoorani. 04549-287521 Tirumangalam Government Primary Health Madhusuthanan 9894448652 6. Centre, Kallikudi. C. 04549-278518 Government Primary Health Anantha Jothi 9444718441 7. Centre, Chellampatti. R.S. 04552-243281 Usilampatti Government Primary Health 9894187265 8. Centre, Krishnananth P. 94430 92915 Thottappanayakkanur. Government Primary Health Muthudurai 9443432352 9. Centre, T.Kallupatti. Kannan P S 04549- 270733 Peraiyur Government Primary Health 9790194039 10. Susee Pradeep S. Centre, Elumalai. 954552-246319 Government Primary Health Thangavalli R. 9609610999 11. Tiruparankundram Centre, 0452-2485046 Tirupparankundram . Government Primary Health Pandiaraj K.R. 9843667880 12. Centre, Vellalur. 0452-2427545 Melur Government Primary Health Nagadeepa.P 9940968712 13. Centre, Karunkalakudi. 04544-250301 MADURAI HUD - BLOCK MEDICAL OFFICERS PHONE NUMBERS Sl. Cell Phone Present Station Name of the Doctor No. Nos. 1 Kallandhiri Beatricejasminejeyathi 9842010469 2 Alanganallur Dhanasekaran.M.R. 9597950013 3 Katchaikatti Hariprasath V.R. 9894821775 4 Karungalakudi Shanmugaperumal P. 9345207554 5 Vellalore Jeyalakshmi C. 9486362998 6 Thirupparankundram Sivakumar 9894661655 7 Kalligudi Rajasekara.K 9443951740 8 T.Kallupatti Pandiarajan V. 9443044649 9 Chekkanoorani Umamaheswari.A.S 9944831316 10 Chellampatti Rajkaboor.R 9894436880 11 Doddappanayakkanoor Suseela.M 9443092915 12 Samayanallur Saravanan C. 9843939671 13 Elumalai Viswanathaprabhu.H 9443586492 MADURAI HUD - INCHARGE MEDICAL OFFICERS PHONE NUMBERS Cell Phone Sl. -

Government of India
இதிய அர GOVERNMENT OF INDIA இதிய வானிைல ஆ ைற INDIA METEOROLOGICAL மடல வானிைல ஆ ைமய DEPARTMENT 6, காி சாைல, ெசைன - 600006 Regional Meteorological Centre ெதாைலேபசி : 044- 28271951. No. 6, College Road, Chennai–600006 Phone: 044- 28271951. WEEKLY WEATHER REPORT FOR TAMILNADU, PUDUCHERRY & KARAIKAL FOR THE WEEK ENDING 29 September 2021 / 07 ASVINA 1943 (SAKA) SUMMARY OF WEATHER Week’s Rainfall: Large Excess Coimbatore, Kanyakumari, Karur, Madurai, Sivagangai and Thoothukudi districts and Puducherry. Excess Perambalur, Ranipet, Tirunelveli, Tiruvarur, Tiruchirapalli and Villupuram districts. Normal Ariyalur, Cuddalore, Nilgiris, Pudukottai, Salem, Theni, Tirupathur, Tiruppur and Vellore districts. Deficient Chengalpattu, Dindigul, Erode, Kallakurichi, Namakkal, Thenkasi, Tiruvannamalai and Virudhunagar districts. Large Chennai, Dharmapuri, Kanchipuram, Krishnagiri, Mayiladuthurai, Deficient Nagapattinam, Ramanathapuram, Thanjavur and Tiruvallur districts. No rain Karaikal area CHIEF AMOUNTS OF RAINFALL (IN CM): 23.09.2021: Melur (dist Madurai) 7, Tirupuvanam (dist Sivaganga), Pulipatti (dist Madurai) 6 each, Kalavai Aws (dist Ranipet), Tiruppur 5 each, Vembakottai (dist Virudhunagar), Salem (dist Salem) 4 each, Kallakurichi (dist Kallakurichi), Yercaud (dist Salem), Vembakkam (dist Tiruvannamalai), Kariyapatti (dist Virudhunagar) 3 each, Kovilpatti Aws (dist Toothukudi), Sivaganga (dist Sivaganga), Arakonam (dist Ranipet), Vedaranyam (dist Nagapattinam), Rasipuram (dist Namakkal), Thandarampettai (dist Tiruvannamalai), Vaippar (dist Toothukudi), -
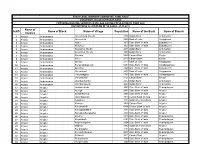
S.NO Name of District Name of Block Name of Village Population Name
STATE LEVEL BANKERS' COMMITTEE, TAMIL NADU CONVENOR: INDIAN OVERSEAS BANK PROVIDING BANKING SERVICES IN VILLAGE HAVING POPULATION OF OVER 2000 DISTRICTWISE ALLOCATION OF VILLAGES -01.11.2011 Name of S.NO Name of Block Name of Village Population Name of the Bank Name of Branch District 1 Ariyalur Andiamadam Anikudichan (South) 2730 Indian Bank Andimadam 2 Ariyalur Andiamadam Athukurichi 5540 Bank of India Alagapuram 3 Ariyalur Andiamadam Ayyur 3619 State Bank of India Edayakurichi 4 Ariyalur Andiamadam Kodukkur 3023 State Bank of India Edayakurichi 5 Ariyalur Andiamadam Koovathur (North) 2491 Indian Bank Andimadam 6 Ariyalur Andiamadam Koovathur (South) 3909 Indian Bank Andimadam 7 Ariyalur Andiamadam Marudur 5520 Canara Bank Elaiyur 8 Ariyalur Andiamadam Melur 2318 Canara Bank Elaiyur 9 Ariyalur Andiamadam Olaiyur 2717 Bank of India Alagapuram 10 Ariyalur Andiamadam Periakrishnapuram 5053 State Bank of India Varadarajanpet 11 Ariyalur Andiamadam Silumbur 2660 State Bank of India Edayakurichi 12 Ariyalur Andiamadam Siluvaicheri 2277 Bank of India Alagapuram 13 Ariyalur Andiamadam Thirukalappur 4785 State Bank of India Varadarajanpet 14 Ariyalur Andiamadam Variyankaval 4125 Canara Bank Elaiyur 15 Ariyalur Andiamadam Vilandai (North) 2012 Indian Bank Andimadam 16 Ariyalur Andiamadam Vilandai (South) 9663 Indian Bank Andimadam 17 Ariyalur Ariyalur Andipattakadu 3083 State Bank of India Reddipalayam 18 Ariyalur Ariyalur Arungal 2868 State Bank of India Ariyalur 19 Ariyalur Ariyalur Edayathankudi 2008 State Bank of India Ariyalur 20 Ariyalur