Evaluation of the Physiographic Method for the Tasman Region
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Motupipi-Takaka Terraces and Plains Ecosystem Plant Lists
MOTUPIPI – TAKAKA TERRACES & PLAINS ECOSYSTEM NATIVE PLANT RESTORATION LIST Flats east of the Takaka and Motupipi River floodplains, extending from Pohara inland up the Takaka valley to Uruwhenua and backed in the east by limestone and marble Locality: hill country. Outliers west of Takaka River include terraces of Go-Ahead Creek and those north of One-Spec Creek. Plains and terraces, in the north reaching 40m above sea level and gently dipping to Topography: the north-west, and inland rising up to 80m above sea level. Low-lying towards Motupipi Inlet and coast. Alluvial, sandy and silty loams of moderate fertility derived from a range of sedimentary rocks, marble, granites and schist. Overlying unweathered glacial outwash gravels. Soils and Geology: Mostly well-drained except along low-gradient, meandering water courses and behind strip of sandy coast at Motupipi-Clifton where there are swamp deposits. Not drought- prone. High sunshine hours; frosts mild to moderate; mild annual temperatures. Climate: Rainfall 1600mm on the coast to 2600mm inland. Coastal Influence: Between Motupipi Estuary and Pohara up to ½ km inland. Mixed podocarp-broadleaf forest, especially tōtara, mataī, tītoki and northern rātā on Original Vegetation: the drier sites and kāhikatea and pukatea on the less well-drained sites. Wetlands in low-lying areas. No original vegetation remaining. A few small and isolated secondary forest and Human Modification: treeland remnants persist as well as numerous scattered individual trees, particularly tōtara. [Refer to the Ecosystem Restoration map showing the colour-coded area covered by this list.] KEY TYPE OF FOOD PROVIDED FOR PLANTING RATIO PLANT PREFERENCES BIRDS AND LIZARDS Early Stage plants are able to Wet, Moist, Dry, Sun, Shade, Frost, Saline establish in open sites and can act as F = Fruit/seeds a nursery for later stage plants by 1 = prefers or tolerates providing initial cover. -

Moutere Gravels
LAND USE ON THE MOUTERE GRAVELS, I\TELSON, AND THE DilPORTANOE OF PHYSIC.AL AND EOONMIC FACTORS IN DEVJt~LOPHTG THE F'T:?ESE:NT PATTERN. THESIS FOR THE DEGREE OF MASTER OF ARTS ( Honours ) GEOGRAPHY UNIVERSITY OF NEW ZEALAND 1953 H. B. BOURNE-WEBB.- - TABLE OF CONTENTS. CRAFTER 1. INTRODUCTION. Page i. Terminology. Location. Maps. General Description. CH.AFTER 11. HISTORY OF LAND USE. Page 1. Natural Vegetation 1840. Land use in 1860. Land use in 1905. Land use in 1915. Land use in 1930. CHA.PrER 111. PRESENT DAY LAND USE. Page 17. Intensively farmed areas. Forestry in the region. Reversion in the region. CHA.PrER l V. A NOTE ON TEE GEOLOGY OF THE REGION Page 48. Geological History. Composition of the gravels. Structure and surface forms. Slope. Effect on land use. CHA.mm v. CLIMATE OF THE REGION. Page 55. Effect on land use. CRAFTER Vl. SOILS ON Tlffi: MGm'ERE GRAVELS. Page 59. Soil.tYJDes. Effect on land use. CHAPrER Vll. ECONOMIC FACTORS WrIICH HAVE INFLUENCED TEE LAND USE PATTERN. Page 66. ILLUSTRATIONS AND MAPS. ~- After page. l. Location. ii. 2. Natu.ral Vegetation. i2. 3. Land use in 1905. 6. Land use regions and generalized land use. 5. Terraces and sub-regions at Motupiko. 27a. 6. Slope Map. Folder at back. 7. Rainfall Distribution. 55. 8. Soils. 59. PLATES. Page. 1. Lower Moutere 20. 2. Tapawera. 29. 3. View of Orcharding Arf;;a. 34a. 4. Contoured Orchard. 37. 5. Reversion and Orchards. 38a. 6. Golden Downs State Forest. 39a. 7. Japanese Larch. 40a. B. -

MUSIC MAN Community 3-7
October 2017 Inside this issue: MUSIC MAN Community 3-7 Recreation 9-11 Arts and Crafts 13 Moutere Youth 15 Food 17 Animals 19-21 Gardening 22 Health & Wellbeing 23 Trade & Services 26 directory Recycled materials are a perfect basis for Lawrie Feely’s stringed instruments Special points of interest: and stored for 30 years, and I’ve used it in a lot of my L O C A L L I V E S instruments. Each wood has a different sound.” His favourite is the strum stick—a portable version of Every Friday Sharing table the dulcimer that can be played like a guitar instead of MHCC Eight-string island ukuleles, strum sticks and mountain on a table. “Backpackers love them,” he says. Also dulcimers are everywhere to be seen in Lawrie Feely’s popular is a stringed instrument that can be played by workshop. Created from recycled venetian blinds, fruit anyone who’s capable of a single finger tune on the 14 October: The Andrew bowls, tabletops and bedheads, each one looks and piano. “You can make music out of anything,” says London Trio—page 11 sounds unique. Lawrie, pulling out a ‘tin-canjo’ with a decorative biscuit- Lawrie has been making instruments since going to a tin body to prove his point. 70th birthday party down South and playing along with a 20 October: Musical When he’s not making instruments, Lawrie can be found group of ladies from the marae on the ukulele. “Next repairing horse gear, such as covers, bridles and saddle bingo—page 10 day, I took some photos and measurements and made strapping. -

The Nelson-Marlborough Region
SCIENCE & RESEARCH SERIES NO.43 ARCHAEOLOGICAL RESEARCH AND MANAGEMENT STRATEGY: THE NELSON-MARLBOROUGH REGION by Aidan J. Challis Published by Head Office, Department of Conservation, P O Box 10-420, Wellington, New Zealand ISSN 0113-3713 ISBN 0-478-01334-5 © 1991, Department of Conservation National Library of New Zealand Cataloguing-in-Publication data Challis, Aidan J. (Aidan John), 1948- Archaeological research and management strategy : the Nelson-Marlborough Region / by Aidan J. Challis. Wellington, N.Z. : Head Office, Dept. of Conservation, c1991. 1 v. (Science & research series, 0113-3713 ; no. 43) Includes bibliographical references. ISBN 0-478-01334-5 1. Historic sites--New Zealand--Nelson-Marlborough Region— Conservation and restoration. 2. Excavations (Archaeology)— New Zealand--Nelson-Marlborough Region. 3. Maori (New Zealand people)--New Zealand--Nelson-Marlborough Region--Antiquities. 4. Nelson-Marlborough Region (N.Z.)--Antiquities. I. New Zealand.Dept. of Conservation. II. Title. III. Series: Science & research series ; no. 43. 363.6909935 Keywords: archaeological zones, Golden Bay, Granite Coast, Mineral Belt, Motueka River, Moutere Hills, site management, site protection, site significance, Clarence, D'Urville, Hundalee, Inland Marlborough, Kaikoura, Nelson, North-West Nelson, Richmond, Sounds, Wairau, NZMS260/P25, NZMS260/P26, NZMS260/N27, NZMS260/N25, NZMS260/M25, NZMS260/N26, NZMS262/9, NZMS260/O28 CONTENTS ABSTRACT 1 1 INTRODUCTION 1 2 THE PROGRESS OF ARCHAEOLOGICAL RESEARCH 2 3 SUMMARY SYNTHESIS OF PRE-EUROPEAN -
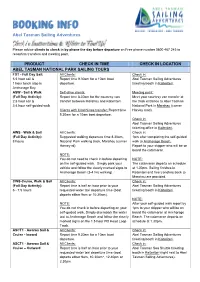
Abel Tasman Sailing Adventures
Abel Tasman Sailing Adventures Please advise clients to check in by phone the day before departure on Free phone number 0800 467 245 to reconfirm transfers and meeting point. PRODUCT CHECK IN TIME CHECK IN LOCATION ABEL TASMAN NATIONAL PARK SAILING TOURS FDT - Full Day Sail: All Clients: Check in: 5.5 hour sail & Report time 9.30am for a 10am boat Abel Tasman Sailing Adventures 1 hour lunch stop in departure. ticketing booth in Kaiteriteri. Anchorage Bay ASW - Sail & Walk Self-drive clients: Meeting point: (Full Day Activity): Report time 8.30am for the courtesy van Meet your courtesy van transfer at 2.5 hour sail & transfer between Marahau and Kaiteriteri the track entrance to Abel Tasman 3-4 hour self-guided walk National Park in Marahau (corner Clients with Coachlines transfer: Report time Harvey road). 9.30am for a 10am boat departure. Check in: Abel Tasman Sailing Adventures ticketing office in Kaiteriteri. AWS - Walk & Sail All Clients: Check in: (Full Day Activity): Suggested walking departure time 8.30am, 1pm after completing the self-guided 8 hours National Park walking track, Marahau (corner walk to Anchorage Beach. Harvey rd). Report to your skipper who will be on board the catamaran. NOTE: You do not need to check in before departing NOTE: on the self-guided walk. Simply park your The catamaran departs on schedule vehicle and follow the clearly marked signs to at 1.30pm. Sailing finishes in Anchorage Beach (3-4 hrs walking). Kaiteriteri and free transfers back to Marahau are provided. CWS-Cruise, Walk & Sail All Clients: Check in: (Full Day Activity): Report time is half an hour prior to your Abel Tasman Sailing Adventures 6 - 7.5 hours requested water taxi departure time (boat ticketing booth in Kaiteriteri. -

Feasibility of Restoring Tasman Bay Mussel Beds
Feasibility of restoring Tasman Bay mussel beds Prepared for Nelson City Council May 2012 29 June 2012 11.52 a.m. Authors/Contributors : Sean Handley Stephen Brown For any information regarding this report please contact: Sean Handley Marine Ecologist Nelson Marine Ecology and Aquaculture +64-3-548 1715 [email protected] National Institute of Water & Atmospheric Research Ltd 217 Akersten Street, Port Nelson PO Box 893 Nelson 7040 New Zealand Phone +64-3-548 1715 Fax +64-3-548 1716 NIWA Client Report No: NEL2012-013 Report date: May 2012 NIWA Project: ELF12243 © All rights reserved. This publication may not be reproduced or copied in any form without the permission of the copyright owner(s). Such permission is only to be given in accordance with the terms of the client’s contract with NIWA. This copyright extends to all forms of copying and any storage of material in any kind of information retrieval system. Whilst NIWA has used all reasonable endeavours to ensure that the information contained in this document is accurate, NIWA does not give any express or implied warranty as to the completeness of the information contained herein, or that it will be suitable for any purpose(s) other than those specifically contemplated during the Project or agreed by NIWA and the Client. 29 June 2012 11.52 a.m. Contents Executive summary .............................................................................................................. 5 1 Introduction ................................................................................................................ -

Waimea Inlet Restoration Information for Communities on Best Practice Approaches CONTENTS
Waimea Inlet restoration Information for communities on best practice approaches CONTENTS 1. Purpose 1 2. Context 1 2.1 Why restore Waimea Inlet’s native ecosystems? 1 2.2 Long-term benefits of restoration 3 2.3 Threats to Waimea Inlet 3 2.4 ‘Future proofing’ for climate change 4 3. Legal considerations 4 4. Ways to get involved 5 4.1 Join an existing project 5 4.2 Set up your own project 5 4.3 Other ways to contribute 6 5. Basic principles for restoration projects 6 5.1 Habitat restoration and amenity planting values 6 5.2 Ecosourcing 7 5.3 Ecositing 7 6. Project planning and design 8 6.1 Restoration plan and objectives 8 6.2 Health and safety 9 6.3 Baseline surveys of the area’s history, flora, fauna and threats 9 7. Implementation – doing the restoration work 12 7.1 The 5 stages of restoration planting 12 7.2 How to prepare your site 14 7.3 How to plant native species 17 7.4 Cost estimates for planting 19 7.5 Managing sedimentation 19 7.6 Restoring whitebait habitat 19 7.7 Timelines 20 7.8 Monitoring and follow-up 20 Appendix 1: Native ecosystems and vegetation sequences in Waimea Inlet’s estuaries and estuarine margin 21 Appendix 2: Valuable riparian sites in Waimea Inlet for native fish, macroinvertebrates and plants 29 Appendix 3: Tasman District Council list of Significant Natural Areas for native species in Waimea Inlet estuaries, margins and islets 32 Appendix 4: Evolutionary and cyclical nature of community restoration projects 35 Appendix 5: Methods of weed control 36 Appendix 6: Further resources 38 1. -

Nitrate Sources and Residence Times of Groundwater in the Waimea Plains, Nelson
Journal of Hydrology (NZ) 50 (2): 313-338 2011 © New Zealand Hydrological Society (2011) Nitrate sources and residence times of groundwater in the Waimea Plains, Nelson Michael K. Stewart1, Glenn Stevens2, Joseph T. Thomas2, Rob van der Raaij3, Vanessa Trompetter3 1 Aquifer Dynamics & GNS Science, PO Box 30368, Lower Hutt, New Zealand. Corresponding author: [email protected] 2 Tasman District Council, Private Bag 4, Richmond, New Zealand 3 GNS Science, PO Box 30368, Lower Hutt, New Zealand Abstract the various wells. The timing of the derived Nitrate concentrations exceeding Ministry of nitrate input history shows that both the Health potable limits (11.3 mg/L nitrate-N) diffuse sources and the point source were have been a problem for Waimea Plains present from the 1940s, which is anecdotally groundwater for a number of years. This work the time from which there were increased uses nitrogen isotopes to identify the input nitrate sources on the plains. The large sources of the nitrate. The results in relation piggery was closed in the mid-1980s. to nitrate contours have revealed two kinds Unfortunately, major sources of nitrate of nitrate contamination in Waimea Plains (including the piggery) were located on groundwater – diffuse contamination in the the main groundwater recharge zone of the eastern plains area (in the vicinity and south plains in the past, leading to contamination of Hope) attributed to the combined effects of the Upper and Lower Confined Aquifers. of the use of inorganic fertilisers and manures The contamination travelled gradually for market gardening and other land uses, northwards, affecting wells on the scale of and point source contamination attributed to decades. -
Conservation Campsites South Island 2019-20 Nelson
NELSON/TASMAN Note: Campsites 1–8 and 11 are pack in, pack out (no rubbish or recycling facilities). See page 3. Westhaven (Te Tai Tapu) Marine Reserve North-west Nelson Forest Park 1 Kahurangi Marine Takaka Tonga Island Reserve 2 Marine Reserve ABEL TASMAN NATIONAL PARK 60 3 Horoirangi Motueka Marine KAHURANGI Reserve NATIONAL 60 6 Karamea PARK NELSON Picton Nelson Visitor Centre 4 6 Wakefield 1 Mount 5 6 Richmond Forest Park BLENHEIM 67 6 63 6 Westport 7 9 10 Murchison 6 8 Rotoiti/Nelson Lakes 1 Visitor Centre 69 65 11 Punakaiki NELSON Marine ReservePunakaiki Reefton LAKES NATIONAL PARK 7 6 7 Kaikōura Greymouth 70 Hanmer Springs 7 Kumara Nelson Visitor Centre P Millers Acre/Taha o te Awa Hokitika 73 79 Trafalgar St, Nelson 1 P (03) 546 9339 7 6 P [email protected] Rotoiti / Nelson Lakes Visitor Centre Waiau Glacier Coast P View Road, St Arnaud Marine Reserve P (03) 521 1806 Oxford 72 Rangiora 73 0 25 50 km P [email protected] Kaiapoi Franz Josef/Waiau 77 73 CHRISTCHURCH Methven 5 6 1 72 77 Lake 75 Tauparikākā Ellesmere Marine Reserve Akaroa Haast 80 ASHBURTON Lake 1 6 Pukaki 8 Fairlie Geraldine 79 Hautai Marine Temuka Reserve Twizel 8 Makaroa 8 TIMARU Lake Hāwea 8 1 6 Lake 83 Wanaka Waimate Wanaka Kurow Milford Sound 82 94 6 83 Arrowtown 85 6 Cromwell OAMARU QUEENSTOWN 8 Ranfurly Lake Clyde Wakatipu Alexandra 85 Lake Te Anau 94 6 Palmerston Te Anau 87 8 Lake Waikouaiti Manapouri 94 1 Mossburn Lumsden DUNEDIN 94 90 Fairfield Dipton 8 1 96 6 GORE Milton Winton 1 96 Mataura Balclutha 1 Kaka Point 99 Riverton/ INVERCARGILL Aparima Legend 1 Visitor centre " Campsite Oban Stewart Island/ National park Rakiura Conservation park Other public conservation land Marine reserve Marine mammal sanctuary 0 25 50 100 km NELSON/TASMAN Photo: DOC 1 Tōtaranui 269 This large and very popular campsite is a great base for activities; it’s a good entrance point to the Abel Tasman Coast Track. -

THE NEW ZEALAND GAZETTE No. 79
2002 THE NEW ZEALAND GAZETTE No. 79 NELSON CONSERVANCY-Continued Reg. Operator Postal Address Location of Mill No. 303 Baigent, H., and Sons Ltd. P.O. Box 97, Nelson .. Wakefield 221 Barnes, T. H., and Co. Ltd. Murphy's Road, Blenheim Okoha 155 Bastin, W., and Sons Edward Street, Wakefield Maud Creek 112 Benara Timber Co. Ltd. P.O. Box 10, Nelson .. Mangarakau 199 Blackadder, W. D. .. Rahu, Reefton Rahu 152 Brown Creek Sawmilling Co. Ltd. P.O. Box 14, Ikamatua Ikamatua 286 Bruning, N. C. R.M.D., Takaka Waitapu 290 Bryant Bros. P.O. Box 240, Blenheim Canvastown 8 Chamberlain Construction Ltd. P.O. Box 291, Nelson Korere 161 Chandler Bros. Care of P.O. Box 63, Westport Mokihinui 229 Couper Bros. Rai Valley Marlborough Rai Valley 213 Crispin, A. C. R. Havelock .. Havelock 178 Cronadun Timbers Ltd. P.O. Box 234, Greymouth Larry's Creek (1) 24 De Boo Bros. Rai Valley .. Carluke 156 Deck Bros. Riwaka R.M.D. 3, Motueka Riwaka 173 Donnelly Milling Co. Ltd. Care of P.O. Box 10, Nelson " Hope 277 Duncan, J. W. C. and N. H. Tapawera R.D. 2, Wakefield .. Tapawera 200 Eggers, R. T., and Sons Ltd. R.D. No.2, Upper Moutere, Nelson Harakeke 282 Farrington, L. and M. Mistlands, Tutaki R.D., Murchison Tutaki 292 Fleming Bros. Howard Post Office, Nelson Howard 257 Fleming, W. T. A. Waller Street, Murchison Murchison 183 Gibson, B. R. P.O. Box 184, Nelson Rai Valley 291 Gordon,· R. K. P.O. Box 34, Murchison Shenandoah 274 Granger Bros. -

52510 Tasman Golf Map A3 Flyer.Indd
Free Official Waahi Taakaro Golf Club Nelson Golf Club Nelson Golf Map Located in the peaceful Maitai Valley just a few minutes from Situated next to Nelson Airport with impressive sea and central Nelson City, this 9 hole picturesque course provides a mountain views, this is one of the few true links courses in the surprisingly stern test, with golfers having to negotiate a river country and is rated in the top 40 by NZ Golf Digest. The 18 hole and a steep hill on their way round. layout has hosted many NZ Amateur and other championship events and is renowned for its superb greens and bunkering. Address: 336 Maitai Valley Road, Nelson Phone: 03 548 7301 Golf Shop, Address: 38 Bolt Road, Tahunanui, Nelson 03 548 7771 Club Phone 03 548 5028 Golf Shop 03 544 8420 Club www.nelsongolf.co.nz Greenacres Golf Club Totaradale Golf Club Set on an island, Greenacres Golf Club is renowned as one of This pleasant and well-tended 9-hole course, situated a few the best all-weather courses in the region, offering magnificent minutes from Wakefield, meanders around some gentle hills scenery and tranquil surroundings. The beautifully maintained 18 populated with some impressive trees and with lovely views hole layout, rated one of the top 40 courses in New Zealand, is looking down over Pigeon Valley. conveniently located on the outskirts of Richmond and a just a Nelson / Tasman short drive from Nelson airport. Address: 147 Pigeon Valley Road, Wakefield Phone: 03 541 8030 Address: 4 Barnett Ave, Best Island, Richmond www.totaradalegolf.co.nz Golf Trails Phone 03 544 6441 Golf Shop 03 544 8420 Club www.tasmangolf.co.nz www.greenacresgolfclub.co.nz Tasman Golf Club Golden Downs Golf Club Murchison Golf Club Tasman Golf Club at Kina Cliffs aims to offer members and This charming 9 hole country course about 45 minutes drive A relaxed and rustic 9 hole course nestled next to the Buller visitors an exceptional golfing and scenic experience. -

Christine Prasad 2020
DEVELOPMENT OF GEOTECHNICAL GROUND MODELS FOR SLOPE INSTABILITY, EASTERN SIDE, TAKAKA HILL, TASMAN DISTRICT A thesis Submitted in partial fulfilment of the requirements for the degree of Master of Science in Engineering Geology at the University of Canterbury By Christine Prasad 2020 Abstract State Highway 60 (SH60) over Takaka Hill in Tasman District has a long history of damage and closure from rainfall-triggered slope failures. Extreme rainfall from Ex-tropical cyclone Gita on 20th February 2018 triggered numerous translational soil slides on the southern side of Takaka Hill resulting in a series of debris flows that damaged SH60 near Riwaka. Scour erosion of road seal, damage to culverts, and failure of embankment sections led to complete closure of the road for five days, preventing all road access to the Takaka- Collingwood region. The road continues to be reduced to a single lane with traffic control more than two years after the event as repairs are ongoing. A programme of geomorphic mapping, geophysical profiling, and both in-situ and laboratory geotechnical testing was carried out to characterise the 2018 debris flows and investigate the hazard from rainfall-triggered slope failure and debris flows on Takaka Hill. Three stream catchments, on the lower slopes of the southern side of Takaka Hill, were the focus of this investigation. These are underlain by differing bedrock: granite, schist and a basic igneous suite. Historical records indicate an approximately 30-year recurrence for major events resulting in road closure for ≥ 5 days. The main types of rainfall-triggered failures are culvert blockage, shallow soil slides and debris flows.