VU Research Portal
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

UC Davis Dermatology Online Journal
UC Davis Dermatology Online Journal Title Proposed classification for koebner, wolf isotopic, renbok, koebner nonreaction, isotopic nonreaction & other related phenomen. Permalink https://escholarship.org/uc/item/96s656b4 Journal Dermatology Online Journal, 20(11) Authors Kannangara, Ajith P Yosipovitch, Gil Fleischer Jr., Alan B Publication Date 2014 DOI 10.5070/D32011024682 License https://creativecommons.org/licenses/by-nc-nd/4.0/ 4.0 eScholarship.org Powered by the California Digital Library University of California Volume 20 Number 11 November 2014 Commentary Proposed classification for koebner, wolf isotopic, renbok, koebner nonreaction, isotopic nonreaction & other related phenomen. Ajith P. Kannangara MD1, Gil Yosipovitch MD PhD2, Alan B. Fleischer Jr MD3 Dermatology Online Journal 20 (11): 12 1Base Hospital Balapitiya, Ministry of Health, Sri Lanka 2Department of Dermatology, Temple University School of Medicine, Philadelphia, USA 3Department of Dermatology, Wake Forest University School of Medicine; Winston-Salem, North Carolina, USA Correspondence: Ajith P. Kannangara, M.D Base Hospital Balapitiya, Ministry of Health, Sri Lanka. E-mail: [email protected] Abstract Students of skin diseases have long noted a variety of disease responses and non-responses to trauma and the presence of structural abnormalities. This article will review the series of these responses including: Koebner phenomenon, Wolf isotopic response, Renbök response, Koebner nonreaction, isotopic nonreaction, and other related skin reactions. Because most of these reported phenomena have similar morphological features the diagnosis is often made on the basis of differences in the clinical presentation. Note that some of the cutaneous reactions of similar phenomena have been described using varied nomenclature, further adding to the confusion. -

Heinrich Koebner and His Phenomenon
Patient Preferences in Dermatologist Attire Original Investigation Research 3. Griffin SJ, Kinmonth AL, Veltman MW, Gillard S, 6. Petrilli CM, Mack M, Petrilli JJ, Hickner A, Saint S, 9. Maruani A, Léger J, Giraudeau B, et al. Effect of Grant J, Stewart M. Effect on health-related Chopra V. Understanding the role of physician attire physician dress style on patient confidence. JEur outcomes of interventions to alter the interaction on patient perceptions: a systematic review of the Acad Dermatol Venereol. 2013;27(3):e333-e337. between patients and practitioners: a systematic literature: targeting attire to improve likelihood of 10. Gamble RG, Hay AA, Dunn JH, Dellavalle RP. review of trials. Ann Fam Med. 2004;2(6):595-608. rapport (TAILOR) investigators. BMJ Open.2015;5 Dermatologists wearing white coats on practice 4. Barbosa CD, Balp MM, Kulich K, Germain N, (1):e006578. websites: current trends. Dermatol Reports. 2011;3 Rofail D. A literature review to explore the link 7. Thomas MW, Burkhart CN, Lugo-Somolinos A, (1):e6. between treatment satisfaction and adherence, Morrell DS. Patients’ perceptions of physician attire 11. Burden M, Cervantes L, Weed D, Keniston A, compliance, and persistence. Patient Prefer in dermatology clinics. Arch Dermatol. 2011;147(4): Price CS, Albert RK. Newly cleaned physician Adherence. 2012;6:39-48. 505-506. uniforms and infrequently washed white coats have 5. Rehman SU, Nietert PJ, Cope DW, Kilpatrick AO. 8. Kanzler MH, Gorsulowsky DC. Patients’ attitudes similar rates of bacterial contamination after an What to wear today? effect of doctor’s attire on the regarding physical characteristics of medical care 8-hour workday: a randomized controlled trial. -

The Koebner Phenomenon and Breast Reconstruction: Psoriasis Eruption Along the Surgical Incision
COPYRIGHT PULSUS CASEGROUP REPORT INC. – DO NOT COPY The Koebner phenomenon and breast reconstruction: Psoriasis eruption along the surgical incision Noor Alolabi BHSc1, Colin P White MD2, Arianna Dal Cin MD FRCSC3 N Alolabi, CP White, A Dal Cin. The Koebner phenomenon and Le phénomène de Koebner et la reconstruction breast reconstruction: Psoriasis eruption along the surgical incision. mammaire : une éruption de psoriasis le long de Can J Plast Surg 2011;19(4):143-144. l’incision chirurgicale The present report describes a recent case of recurrent infection in a breast Le présent rapport décrit un cas récent d’infection récurrente chez une reconstruction patient with a history of psoriasis. Following surgery, the patiente ayant subi une reconstruction mammaire et ayant des antécédents patient developed psoriatic plaques along the incision scars. This phenom- de psoriasis. Après l’opération, la patiente a développé des plaques de enon is known as Koebnerization, and has been found to affect surgical psoriasis le long des cicatrices d’incision. Il s’agit du phénomène de incisions. Cases of psoriatic patients being denied surgical procedures Koebner, qui s’attaque aux incisions chirurgicales. On a déjà rendu compte because of their inherent risk to Koebnerize have been previously reported. de cas de patients psoriasiques à qui on avait refusé des interventions Similarly, such patients have been denied surgical procedures because of chirurgicales en raison du risque inhérent de phénomène de Koebner ou de their increased risk of infection. The present case and literature review on leur risque accru d’infection. Les auteurs exposent le cas ainsi qu’une this subject is described. -

Fenômeno Isomórfico De Koebner
UNIVERSIDADE ESTADUAL DE CAMPINAS FACULDADE DE CIÊNCIAS MÉDICAS GABRIEL VILLAS-BOAS DOS SANTOS TABOSA FENÔMENOS ISOMÓRFICO (DE KOEBNER), PSEUDO-ISOMÓRFICO (PSEUDO- KOEBNER), ISOTÓPICO (DE WOLF), ISOPÁTICO E DISTRITO IMUNOCOMPROMETIDO CUTÂNEO – REVISÃO SISTEMÁTICA CAMPINAS 2019 GABRIEL VILLAS-BOAS DOS SANTOS TABOSA FENÔMENOS ISOMÓRFICO (DE KOEBNER), PSEUDO-ISOMÓRFICO (PSEUDO- KOEBNER), ISOTÓPICO (DE WOLF), ISOPÁTICO E DISTRITO IMUNOCOMPROMETIDO CUTÂNEO – REVISÃO SISTEMÁTICA Dissertação apresentada à Faculdade de Ciências Médicas da Universidade Estadual de Campinas como parte dos requisitos exigidos para obtenção do título de Mestre em Ciências, Área de Concentração em Anatomia Patológica. ORIENTADORA: MARIA LETÍCIA CINTRA ESTE TRABALHO CORRESPONDE À VERSÃO FINAL DA DISSERTAÇÃO DEFENDIDA PELO ALUNO GABRIEL VILLAS-BOAS DOS SANTOS TABOSA E ORIENTADO PELA PROFª. DRª. MARIA LETÍCIA CINTRA. CAMPINAS 2019 Ficha catalográfica Universidade Estadual de Campinas Biblioteca da Faculdade de Ciências Médicas Maristella Soares dos Santos - CRB 8/8402 Tabosa, Gabriel Villas-Boas dos Santos, 1992- T114f Fenômenos isomórfico (de Koebner), pseudo-isomórfico (pseudo-Koebner), isotópico (de Wolf), isopático e distrito imunocomprometido cutâneo - revisão sistemática / Gabriel Villas-Boas dos Santos Tabosa. – Campinas, SP : [s.n.], 2019. Orientador: Maria Letícia Cintra. Dissertação (mestrado) – Universidade Estadual de Campinas, Faculdade de Ciências Médicas. 1. Revisão sistemática. 2. Fenômeno de Wolf. 3. Koebner. 4. Fenômeno isopático. 5. Distrito imunocomprometido -

Resident's Page
Resident’s Page The isomorphic phenomenon of Koebner Devinder Mohan Thappa Department of Dermatology and STD, Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), Pondicherry, India. Address for correspondence: Dr Devinder Mohan Thappa, Professor and Head, Department of Dermatology and STD, JIPMER, Pondicherry - 605006, India. E-mail: [email protected] The Koebner (isomorphic) phenomenon (response) is for National Culture, he presented the phenomenon probably one of the most well known phenomena in that bears his name,4 and 4 years later published a paper dermatology and is named after the man who was the describing his original patient. The mechanism of first to describe it.1 The isomorphic phenomenon is experimentally producing such a reaction was known now well documented in a number of skin diseases. as the Koebner experiment.2 Since the time of Koebner, this phenomenon has been the subject of research by DEFINITION several authors. The Koebner phenomenon is the development of TYPES OF KOEBNER PHENOMENON isomorphic pathologic lesions in the traumatized uninvolved skin of patients who have cutaneous Boyd and Neldner have classified all reported cases of diseases.2 It refers to the fact that in persons with Koebner phenomenon into four different groups:4 certain skin diseases, especially psoriasis, trauma is 1. True isomorphic phenomenon: There appear to followed by new lesions in the traumatized but be three disease processes that display the true otherwise normal skin, and these new lesions are isomorphic response of Koebner: psoriasis, lichen clinically and histopathologically identical to those in planus and vitiligo; the diseased skin.3 2. Pseudoisomorphic phenomenon: The Koebner phenomenon seen in infectious diseases, e.g. -

Koebner Phenomenon Leading to the Formation of New Psoriatic Lesions: Evidences and Mechanisms
Bioscience Reports (2019) 39 BSR20193266 https://doi.org/10.1042/BSR20193266 Review Article Koebner phenomenon leading to the formation of new psoriatic lesions: evidences and mechanisms Yong-Zhi Ji and Shi-Rui Liu Department of Dermatology, Second Hospital of Jilin University, Changchun, China Correspondence: Shi-Rui Liu ([email protected]) Koebner phenomenon refers to the emergence of new psoriatic lesions in the healthy skin re- gions following an injury/trauma to psoriatic patients. The occurrence of psoriatic lesions at unusual areas of the body regions such as on penis, around eyes and on keloids suggest that the Koebner phenomenon may be responsible for these lesions. A number of agents/triggers have been reported to induce the development of new psoriatic lesions in healthy skin ar- eas and these include, tattooing skin, radiations, skin incision, viral infections and striae etc. The different mechanisms that contribute in inducing the development of new psoriatic lesions as Koebernization include the involvement of mast cell-derived inflammatory medi- ators such as tryptase, IL-6, IL-8, IL-17, and IL-36γ. Moreover, an increased expression of nerve growth factor (NGF) and vascular endothelial growth factor (VEGF) also contribute in Koebernization. Apart from these, there is a critical role of α 2 β1 integrins, S100A7 (psori- asin) and S100A15 (koebnerisin), change in the ratio of CD4+/CD8+ T cells, down-regulation of mechanosensitive polycystin 1 protein, decrease in inflammation controlling atypical chemokine receptor 2 (ACKR2), reduced expression of N-methyl-D-aspartate (NMDA) re- ceptors (NMDARs) on the keratinocytes and increase in levels of chemokines (CXCL8 and CCL20) in inducing formation of new psoriatic lesions. -
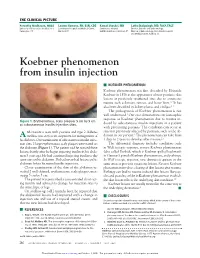
Koebner Phenomenon from Insulin Injection
THE CLINICAL PICTURE Parvathy Madhavan, MBBS Lauren Hamann, RN, BSN, CDE Kamal Shoukri, MD Latha Dulipsingh, MD, FACP, FACE University of Connecticut Health Center, Saint Francis Hospital and Medical Center, Saint Francis Hospital Director, Diabetes and Endocrinology, Farmington, CT Hartford, CT and Medical Center, Hartford, CT Division of Endocrinology, Saint Francis Hospital and Medical Center, Hartford, CT Koebner phenomenon from insulin injection ■ KOEBNER PHENOMENON Koebner phenomenon was first described by Heinrich Koebner in 1876 as the appearance of new psoriatic skin lesions at previously unaffected sites due to cutaneous trauma such as bruises, tattoos, and horse bites.1,2 It has also been described in lichen planus and vitiligo.1–3 The pathogenesis of Koebner phenomenon is not well understood.1 Our case demonstrates an isomorphic response or Koebner phenomenon due to trauma in- Figure 1. Erythematous, scaly plaques 5 cm by 5 cm at subcutaneous insulin injection sites. duced by subcutaneous insulin injections in a patient with preexisting psoriasis. The condition can occur at 68-year-old man with psoriasis and type 2 diabetes sites not previously affected by psoriasis, such as the ab- A mellitus was seen as an outpatient for management of domen in our patient.2 The phenomenon can take from his diabetes. On examination of subcutaneous insulin injec- 3 days to 2 years to develop after trauma.2,3 tion sites, 2 large erythematous scaly plaques were noted on The differential diagnosis includes conditions such the abdomen (Figure 1). The patient said he noticed these as Wolf isotopic response, reverse Koebner phenomenon lesions shortly after he began injecting insulin in his abdo- (also called Renbök, which is Koebner spelled backwards men 5 years ago. -

A Case of Pityriasisrosea Following Cosmetic Shaving
Open Access Full Text Article Journal of Family Medicine A Austin Publishing Group Case Report A Case of Pityriasisrosea Following Cosmetic Shaving James Studdiford1*, Elisabeth P. Collins3, James Studdiford, Daila Pravs1, Brooke Salzman1 and Abstract Jeffrey M. Guarino2 Atypical cases of pityriasisrosea are relatively common; however, there 1Department of Family and Community Medicine, are very few reports of pityriasis rosea exhibiting a koebner-like phenomenon. Thomas Jefferson University, USA We present a case of a 25-year-old healthy male patient with multiple salmon- 2Christiana Care Health Systems, Christiana Hospital, colored, oval-shaped lesions on his abdomen, following along the same area USA which he had previously shaved two weeks prior. The patient was subsequently 3Jefferson Medical College, Thomas Jefferson University, diagnosed with pityriasis rosea presenting in an atypical pattern. It is proposed USA that this atypical presentation of pityriasis rosea was a result of a koebner-like *Corresponding author: James Studdiford, phenomenon triggered by the cosmetic shaving, either caused by a latent human Department of Family and Community Medicine at herpes virus, the putative cause of pityriasis rosea, which was reactivated by Thomas Jefferson University Hospital in Philadelphia, the trauma of the shaving, or by koebnerization of a small herald patch that 1015 Walnut St. Suite 401, Philadelphia, PA 19107, USA, was present before the patient shaved. In general, the authors recommend that Tel: (215) 955-1333; Fax: (215) 955-9158; Email: james. other differentials be excluded before arriving at a diagnosis of atypical pityriasis [email protected] rosea. Yet, with the increased incidence of cosmetic body depilation, it is in a physician’s best interest to keep trauma-induced or koebner-like phenomenon Received: July 15, 2014; Accepted: July 21, 2014; on the differential. -

Red Scaly Rash Following Tattoo Application
PHOTOPHOTO CHALLENGE CHALLENGE Red Scaly Rash Following Tattoo Application Candace M. Broussard-Steinberg, MD; James Lagrew, MD A 26-year-old man presented with a mildly pru- ritic red scaly rash on the right arm of 3 weeks’ duration. He reported having a tattoo placed on previously normal skin on the right lateral arm prior to the development of the rash. Two weeks after receiving the tattoo, he developed scaling and redness of the skin involved in the tattoo. He also had similar papulescopy and plaques over the rest of his body. Physical examination showed well- demarcated, erythematous, scaly papules and plaques following the design of a black-pigmented tattoo on the lateral aspect of the right arm. There alsonot were similar erythematous scaly plaques scat- tered over both arms and the trunk. He denied any Dopain or blister formation of the involved areas. WHAT’S THE DIAGNOSIS? a. allergic contact dermatitis b. irritant contact dermatitis c. isomorphic lichen planus d. isomorphic psoriasis CUTIS e. tattoo granuloma PLEASE TURN TO PAGE E3 FOR THE DIAGNOSIS Dr. Broussard-Steinberg was from the University of South Carolina School of Medicine, Columbia, and currently is from the Department of Dermatology, Indiana University School of Medicine, Indianapolis. Dr. Lagrew was from the Department of Dermatology, Medical University of South Carolina, Charleston, and currently is in private practice, Lexington, Kentucky. The authors report no conflict of interest. Correspondence: Candace M. Broussard-Steinberg, MD, Indiana University School of Medicine, Department of Dermatology, 545 Barnhill Dr, Indianapolis, IN 46202 ([email protected]). E2 I CUTIS® WWW.CUTIS.COM Copyright Cutis 2017. -

MASTER DERMATOLOGISTS* BASED on DISEASES NAMED AFTER THEM by HERMAN GOODMAN, M.D
MASTER DERMATOLOGISTS* BASED ON DISEASES NAMED AFTER THEM By HERMAN GOODMAN, M.D. NEW YORK CITY OR little more than a year, I have lyric and dramatic poetry, sculpture and been collecting material for a architecture. Hippocrates lived in this paper on “Eponyms of Derma- period. The “Father of Recent Medicine” tology,”1 and have especially has icterus gravis named after him as arranged some of the data in concise formHippocratic morbus niger. Calvities Hippo- Fand in chronological order for the Anna ls cratica is the designation of a type of total of Medic al His tory . The names of the baldness and Hippocratic nails are those men cited have come down to us by reason resulting from chronic pulmonary and of the fact that one or more dermatologic cardiac disease. Hippocrates was born on entities or diseases have been named after the Island of Cos about 460 b .c . After them; literally, they are godfathers of the death of his father, he went to Athens dermatology. The names of many master for the study of surgery. The profession of dermatologists who did not have diseases medicine owes to him the art of clinical named after them have been omitted in inspection and observation. The physician’s this article, but I hope at some future date oath has been named the Hippocratic oath, to enlarge on the present study by present- but it is far-fetched to consider him its ing others on the eponyms of dermatologic author. Hippocrates was the founder of anatomy, pathology and therapeutics. Grecian dermatology. His influence per- sisted for over two thousand years. -
Tattoo-Induced Psoriasis
Journal of Medicine and Life Volume 7, Special Issue 2, 2014 Tattoo-induced psoriasis Orzan OA* **, Popa LG**, Vexler ES*, Olaru I*, Voiculescu VM*, Bumbăcea RS* ** *”Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania **Department of Dermatology, ”Elias” Emergency Hospital, Bucharest, Romania Correspondence to: Orzan OA, MD Department of Dermatology, “Elias” Emergency Hospital, Bucharest, 17 Marasti Blvd., District 1, Bucharest Phone: +40 21 316 1600, E-mail: [email protected] Abstract Koebner phenomenon represents the development of several inflammatory skin lesions (psoriasis, lichen planus, vitiligo, etc.) in uninvolved skin following various traumatic insults. The case of a 27-year-old male patient with scalp psoriasis who was referred to our clinic for generalized psoriatic lesions developed two weeks after tattooing his skin at the age of 18 was presented; the case illustrated the possibility of Koebner phenomenon induced by skin tattooing in patients with psoriasis. Keywords: Koebner phenomenon, isomorphic phenomenon, koebnerization, psoriatic lesions, tattoos Introduction Psoriasis is a chronic inflammatory skin disease with an increased epidermal proliferation and it is usually characterized by erythematous, sharp-bordered lesions with silvery scale that can occur in various sites of the body. Although many different factors can trigger the appearance of psoriatic lesions, a certain cause has not yet been established [1]. In patients with this condition, de novo psoriatic lesions often occur at the site of cutaneous trauma. The appearance of these isomorphic lesions is known as the Koebner phenomenon and it occurs in about 25% of people with psoriasis after various traumatic injuries, including tattooing [2]. Case report A 27-year-old patient was referred to our clinic with erythematosus scaly plaques, disseminated on scalp, trunk and upper limbs. -

Seborrheic Dermatitis Induced Koebnerization: a Probable
icine- O ed pe M n l A a c n c r e e s t s n I Oripelaye et al., Intern Med 2017, 7:3 Internal Medicine: Open Access DOI: 10.4172/2165-8048.1000242 ISSN: 2165-8048 Case Report Open Access Seborrheic Dermatitis Induced Koebnerization: A Probable Outcome Determinant in Vitiligo Mufutau Muphy Oripelaye, Fatai Olatunde Olanrewaju, Olaniyi Emmanuel Onayemi and Olayinka Abimbola Olasode Department of Dermatology and Venereology, Obafemi Awolowo University, Ile-Ife, Osun State, Nigeria *Corresponding author: Mufutau Muphy Oripelaye, Department of Dermatology and Venereology, Obafemi Awolowo University, Ile-Ife, Osun State, Nigeria, Tel: 2347030211101 E-mail: [email protected] Received date: June 2, 2017; Accepted date: June 15, 2017; Published date: June 20, 2017 Copyright: © 2017 Oripelaye MM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Abstract Backgrounds: Vitiligo is a pigmentary disorder characterized by depigmentation and absence of melanocyte. It occurs in 0.5-1% of the population and commonly associated with Koebner phenomenon. Koebner phenomenon which is the occurrence of new lesions at site of injury has been described in several dermatoses including vitiligo. It could serve as a useful diagnostic aid and sometimes influence approach to the management of vitiligo when considering surgical intervention. Case report: We report a 64 year old Nigerian who had progressive depigmentation on the scalp, face and palms of 1 year duration. He had prior scaly rashes for eight years which are distributed over the seborrheic areas.