Densities and Viscosities of Binary Liquid Systems of Acetonitrile with Aromatic Ketones at 308.15 K
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Improving Thermodynamic Consistency Among Vapor Pressure, Heat of Vaporization, and Liquid and Ideal Gas Heat Capacities Joseph Wallace Hogge Brigham Young University
Brigham Young University BYU ScholarsArchive All Theses and Dissertations 2017-12-01 Improving Thermodynamic Consistency Among Vapor Pressure, Heat of Vaporization, and Liquid and Ideal Gas Heat Capacities Joseph Wallace Hogge Brigham Young University Follow this and additional works at: https://scholarsarchive.byu.edu/etd Part of the Chemical Engineering Commons BYU ScholarsArchive Citation Hogge, Joseph Wallace, "Improving Thermodynamic Consistency Among Vapor Pressure, Heat of Vaporization, and Liquid and Ideal Gas Heat Capacities" (2017). All Theses and Dissertations. 6634. https://scholarsarchive.byu.edu/etd/6634 This Dissertation is brought to you for free and open access by BYU ScholarsArchive. It has been accepted for inclusion in All Theses and Dissertations by an authorized administrator of BYU ScholarsArchive. For more information, please contact [email protected], [email protected]. Improving Thermodynamic Consistency Among Vapor Pressure, Heat of Vaporization, and Liquid and Ideal Gas Heat Capacities Joseph Wallace Hogge A dissertation submitted to the faculty of Brigham Young University In partial fulfillment of the requirements for the degree of Doctor of Philosophy W. Vincent Wilding, Chair Thomas A. Knotts Dean Wheeler Thomas H. Fletcher John D. Hedengren Department of Chemical Engineering Brigham Young University Copyright © 2017 Joseph Wallace Hogge All Rights Reserved ABSTRACT Improving Thermodynamic Consistency Among Vapor Pressure, Heat of Vaporization, and Liquid and Ideal Gas Heat Capacity Joseph Wallace Hogge Department of Chemical Engineering, BYU Doctor of Philosophy Vapor pressure ( ), heat of vaporization ( ), liquid heat capacity ( ), and ideal gas heat capacity ( ) are important properties for process design and optimization. This work vap Δvap focuses on improving the thermodynamic consistency and accuracy of the aforementioned properties since these can drastically affect the reliability, safety, and profitability of chemical processes. -

Surface Tension Prediction for Liquid Mixtures
AIChE Journal, Volume 44, No. 10, pp. 2324-2332, October 1998 PREPRINT Surface Tension Prediction for Liquid Mixtures Joel Escobedo and G.Ali Mansoori(*) University of Illinois at Chicago (M/C 063) Chicago, IL 60607-7052, USA key words: Surface tension, organic fluids, mixtures, prediction, new mixing rules Abstract Recently Escobedo and Mansoori (AIChE J. 42(5): 1425, 1996) proposed a new expression, originally derived from statistical mechanics, for the surface tension prediction of pure fluids. In this report, the same theory is extended to the case of mixtures of organic liquids with the following expression, 0.37 9 L V 4 = 1-T T exp 0.30066/T + 0.86442 T - m [( r,m) r,m ( r,m r,m ) (P 0,m L,m P 0,m v,m)] 7/3 4 1/4 7/12 = [x x (P / T ) ] (x x T / P ) P0,m { ij i j cij cij P 0,ij } [ij i j cij cij ] 1/2 P0,ij=(1-mij)(P0,iP0,j) where m is the surface tension of the mixture. Tr,m=T/Tc,m and Tc,m is the pseudo-critical temperature of the liquid mixture. and are the equilibrium densities of liquid and L,m v,m L V vapor, respectively. P 0,m and P 0,m are the temperature-independent compound-dependent parameters for the liquid and vapor, respectively. Pc,i , Tc,i and xi are the critical pressure, critical temperature and the mole fraction for component i, respectively. P0,i is the temperature-independent parameter for component i and mij is the unlike interaction parameter. -

Dielectric Constant Chart
Dielectric Constants of Common Materials DIELECTRIC MATERIALS DEG. F CONSTANT ABS RESIN, LUMP 2.4-4.1 ABS RESIN, PELLET 1.5-2.5 ACENAPHTHENE 70 3 ACETAL 70 3.6 ACETAL BROMIDE 16.5 ACETAL DOXIME 68 3.4 ACETALDEHYDE 41 21.8 ACETAMIDE 68 4 ACETAMIDE 180 59 ACETAMIDE 41 ACETANILIDE 71 2.9 ACETIC ACID 68 6.2 ACETIC ACID (36 DEGREES F) 36 4.1 ACETIC ANHYDRIDE 66 21 ACETONE 77 20.7 ACETONE 127 17.7 ACETONE 32 1.0159 ACETONITRILE 70 37.5 ACETOPHENONE 75 17.3 ACETOXIME 24 3 ACETYL ACETONE 68 23.1 ACETYL BROMIDE 68 16.5 ACETYL CHLORIDE 68 15.8 ACETYLE ACETONE 68 25 ACETYLENE 32 1.0217 ACETYLMETHYL HEXYL KETONE 66 27.9 ACRYLIC RESIN 2.7 - 4.5 ACTEAL 21 3.6 ACTETAMIDE 4 AIR 1 AIR (DRY) 68 1.000536 ALCOHOL, INDUSTRIAL 16-31 ALKYD RESIN 3.5-5 ALLYL ALCOHOL 58 22 ALLYL BROMIDE 66 7 ALLYL CHLORIDE 68 8.2 ALLYL IODIDE 66 6.1 ALLYL ISOTHIOCYANATE 64 17.2 ALLYL RESIN (CAST) 3.6 - 4.5 ALUMINA 9.3-11.5 ALUMINA 4.5 ALUMINA CHINA 3.1-3.9 ALUMINUM BROMIDE 212 3.4 ALUMINUM FLUORIDE 2.2 ALUMINUM HYDROXIDE 2.2 ALUMINUM OLEATE 68 2.4 1 Dielectric Constants of Common Materials DIELECTRIC MATERIALS DEG. F CONSTANT ALUMINUM PHOSPHATE 6 ALUMINUM POWDER 1.6-1.8 AMBER 2.8-2.9 AMINOALKYD RESIN 3.9-4.2 AMMONIA -74 25 AMMONIA -30 22 AMMONIA 40 18.9 AMMONIA 69 16.5 AMMONIA (GAS?) 32 1.0072 AMMONIUM BROMIDE 7.2 AMMONIUM CHLORIDE 7 AMYL ACETATE 68 5 AMYL ALCOHOL -180 35.5 AMYL ALCOHOL 68 15.8 AMYL ALCOHOL 140 11.2 AMYL BENZOATE 68 5.1 AMYL BROMIDE 50 6.3 AMYL CHLORIDE 52 6.6 AMYL ETHER 60 3.1 AMYL FORMATE 66 5.7 AMYL IODIDE 62 6.9 AMYL NITRATE 62 9.1 AMYL THIOCYANATE 68 17.4 AMYLAMINE 72 4.6 AMYLENE 70 2 AMYLENE BROMIDE 58 5.6 AMYLENETETRARARBOXYLATE 66 4.4 AMYLMERCAPTAN 68 4.7 ANILINE 32 7.8 ANILINE 68 7.3 ANILINE 212 5.5 ANILINE FORMALDEHYDE RESIN 3.5 - 3.6 ANILINE RESIN 3.4-3.8 ANISALDEHYDE 68 15.8 ANISALDOXINE 145 9.2 ANISOLE 68 4.3 ANITMONY TRICHLORIDE 5.3 ANTIMONY PENTACHLORIDE 68 3.2 ANTIMONY TRIBROMIDE 212 20.9 ANTIMONY TRICHLORIDE 166 33 ANTIMONY TRICHLORIDE 5.3 ANTIMONY TRICODIDE 347 13.9 APATITE 7.4 2 Dielectric Constants of Common Materials DIELECTRIC MATERIALS DEG. -

Equilibrium Critical Phenomena in Fluids and Mixtures
: wil Phenomena I Fluids and Mixtures: 'w'^m^ Bibliography \ I i "Word Descriptors National of ac \oo cop 1^ UNITED STATES DEPARTMENT OF COMMERCE • Maurice H. Stans, Secretary NATIONAL BUREAU OF STANDARDS • Lewis M. Branscomb, Director Equilibrium Critical Phenomena In Fluids and Mixtures: A Comprehensive Bibliography With Key-Word Descriptors Stella Michaels, Melville S. Green, and Sigurd Y. Larsen Institute for Basic Standards National Bureau of Standards Washington, D. C. 20234 4. S . National Bureau of Standards, Special Publication 327 Nat. Bur. Stand. (U.S.), Spec. Publ. 327, 235 pages (June 1970) CODEN: XNBSA Issued June 1970 For sale by the Superintendent of Documents, U.S. Government Printing Office, Washington, D.C. 20402 (Order by SD Catalog No. C 13.10:327), Price $4.00. NATtONAL BUREAU OF STAHOAROS AUG 3 1970 1^8106 Contents 1. Introduction i±i^^ ^ 2. Bibliography 1 3. Bibliographic References 182 4. Abbreviations 183 5. Author Index 191 6. Subject Index 207 Library of Congress Catalog Card Number 7O-6O632O ii Equilibrium Critical Phenomena in Fluids and Mixtures: A Comprehensive Bibliography with Key-Word Descriptors Stella Michaels*, Melville S. Green*, and Sigurd Y. Larsen* This bibliography of 1088 citations comprehensively covers relevant research conducted throughout the world between January 1, 1950 through December 31, 1967. Each entry is charac- terized by specific key word descriptors, of which there are approximately 1500, and is indexed both by subject and by author. In the case of foreign language publications, effort was made to find translations which are also cited. Key words: Binary liquid mixtures; critical opalescence; critical phenomena; critical point; critical region; equilibrium critical phenomena; gases; liquid-vapor systems; liquids; phase transitions; ternairy liquid mixtures; thermodynamics 1. -

Enthalpies of Vaporization of Organic and Organometallic Compounds, 1880–2002
Enthalpies of Vaporization of Organic and Organometallic Compounds, 1880–2002 James S. Chickosa… Department of Chemistry, University of Missouri-St. Louis, St. Louis, Missouri 63121 William E. Acree, Jr.b… Department of Chemistry, University of North Texas, Denton, Texas 76203 ͑Received 17 June 2002; accepted 17 October 2002; published 21 April 2003͒ A compendium of vaporization enthalpies published within the period 1910–2002 is reported. A brief review of temperature adjustments of vaporization enthalpies from temperature of measurement to the standard reference temperature, 298.15 K, is included as are recently suggested reference materials. Vaporization enthalpies are included for organic, organo-metallic, and a few inorganic compounds. This compendium is the third in a series focusing on phase change enthalpies. Previous compendia focused on fusion and sublimation enthalpies. Sufficient data are presently available for many compounds that thermodynamic cycles can be constructed to evaluate the reliability of the measure- ments. A protocol for doing so is described. © 2003 American Institute of Physics. ͓DOI: 10.1063/1.1529214͔ Key words: compendium; enthalpies of condensation; evaporation; organic compounds; vaporization enthalpy. Contents inorganic compounds, 1880–2002. ............. 820 1. Introduction................................ 519 8. References to Tables 6 and 7.................. 853 2. Reference Materials for Vaporization Enthalpy Measurements.............................. 520 List of Figures 3. Heat Capacity Adjustments. ................. 520 1. A thermodynamic cycle for adjusting vaporization ϭ 4. Group Additivity Values for C (298.15 K) enthalpies to T 298.15 K.................... 521 pl 2. A hypothetical molecule illustrating the different Estimations................................ 521 hydrocarbon groups in estimating C ........... 523 5. A Thermochemical Cycle: Sublimation, p Vaporization, and Fusion Enthalpies........... -

How Is the Surface Tension of Various Liquids Distributed Along the Interface Normal?
This document is the Accepted Manuscript version of a Published Work that appeared in final form in J. Phys. Chem. C, copyright © American Chemical Society after peer review and technical editing by the publisher. To access the final edited and published work see http://pubs.acs.org/doi/pdf/10.1021/acs.jpcc.6b09880. How Is the Surface Tension of Various Liquids Distributed along the Interface Normal? Marcello Sega1, Balázs Fábián,2,3 George Horvai,2,4 and Pál Jedlovszky4,5* 1Computational Physics Group, University of Vienna, Sensengasse 8/9, A-1090 Vienna, Austria 2Department of Inorganic and Analytical Chemistry, Budapest University of Technology and Economics, Szt. Gellért tér 4, H-1111 Budapest, Hungary 3 Institut UTINAM (CNRS UMR 6213), Université Bourgogne Franche-Comté, 16 route de Gray, F-25030 Besançon, France 4MTA-BME Research Group of Technical Analytical Chemistry, Szt. Gellért tér 4, H-1111 Budapest, Hungary 5Department of Chemistry, Eszterházy Károly University, Leányka utca 6, H- 3300 Eger, Hungary Running title: Surface Tension Distribution at Liquid Surfaces *Electronic mail: [email protected] Abstract The tangential pressure profile has been calculated across the liquid-vapor interface of five molecular liquids, i.e., CCl4, acetone, acetonitrile, methanol, and water in molecular dynamics simulations using a recently developed method. Since the value of the surface tension is directly related to the integral of this profile, the obtained results can be interpreted in terms of the distribution of the surface tension along the interface normal, both as a function of distance, either from the Gibbs dividing surface or from the capillary wave corrugated real, intrinsic liquid surface, and also in a layerwise manner. -

Acetonitrile
Environmental Health Criteria 154 Acetonitrile lililiiil vile the joint spunsoiship of the United Nations Environment Programme, tl hiitaiiiahiO(Ial Lahoiii Organisation and the World Health Organization THE ENVIRONMENTAL HEALTH CRITERIA SERIES Acetonitrile (No. 154, 1993) 2,4-Dichlorophenoxyacetic acid - environmental Acrolein (No. 127, 1991) aspects (No. 84, 1989) Acrlamide (No. 49, 1985) 1 ,3-Dichtoropropene, 1 ,2-dichloropropane and Acrylonitrile (No. 28, 1983) mixtures (No. 146, 1993) Aged population, principles for evaluating the DDT and its derivatives (No. 9, 1979) effects of chemicals (No. 144, 1992) DDT and its derivatives - environmental aspects Aldicarb (No. 121, 1991) (No. 83, 1989) Aldrin and dieldrin (No. 91, 1989) Deltamethrin (No. 97, 1990) Allethrins (No. 87, 1989) Diaminotduenes (No. 74, 1987) Alphs-cypermethrin (No. 142, 1992) Dichloivos (No. 79, 1988) Ammonia (No. 54, 1986) Diethylhexyl phthalate (No. 131, 1992) Arsenic (No. 18, 1991) Dimethoate (No. 90, 1989) Asbestos and other natural mineral fibres Dimethylformamide (No. 114, 1991) (No. 53, 1986) Dimethyl sulfate (No. 48, 1985) Barium (No. 107, 1990) Diseases of suspected chemical etiology and Benomyl (No. 148, 1993) their prevention, principles of studies on Benzene (No. 150, 1993) (No. 72, 1987) Beryllium (No. 106, 1990) Dithiocarbamate pesticides, ethylenethiourea, and Blotoxins, aquatic (marine and freshwater) propylenethiourea: a general introduction (No. 37, 1984) (No. 78, 1988) Butanols - four isomers (No. 65, 1987) Electromagnetic Fields (No. 137, 1992) Cadmium (No. 134, 1992) Endosulfan (No. 40, 1984) Cadmium environmental aspects (No. 135, 1992) Endrin (No. 130, 1992) Camphechior (No. 45, 1984) Environmental epidemiology, guidelines on Carbamate pesticides: a general introduction studies in (No. 27, 1983) (No. 64, 1986) Epichlorohydrin (No. -

Densities, Refractive Indices and Excess Properties of Binary
Journal of Al-Nahrain University Vol.14 (2), June, 2011, pp.75-85 Science Densities, Refractive Indices and Excess Properties of Binary Mixtures of Acetonitrile with Benzene, Toluene, m-Xylene and Mesitylene at Temperatures from (298.15 to 313.15) K Abeer K. Shams Department of Chemistry, College of Science, Al-Nahrain University. Abstract The densities () and refractive indices (nD) of binary mixtures of acetonitrile with benzene, toluene, m-xylene and mesitylene including those of pure liquids, over the entire composition range expressed by mole fraction x1 of acetonitrile were measured at temperatures (298.15, 303.15, 308.15, and 313.15) K under atmospheric pressure. From the experimental data, the excess molar E volumes (V m ) and the deviations in refractive indices (Δn) were calculated. These results are fit to a Redlich-Kister type polynomial equation. The results indicate the presence of weak interactions between acetonitrile and aromatic hydrocarbon molecules. The deviations in values follow the order toluene < benzene < m-xylene < mesitylene. The results are discussed in terms of the intermolecular interactions. Introduction mixtures for acetonitrile + aromatic hydro- Studies of physicochemical properties of carbons. Afaf H. Absood [6] report densities binary mixtures are of considerable of these binary systems at 303.15K. importance in the fundamental understanding Nevertheless, to our knowledge, no literature of the nature of the interactions between unlike data on densities and refractive indices are molecules. In recent years there has been available for this system. considerable interest in theoretical and Experimental experimental investigations of the excess Chemicals: thermodynamic properties of binary mixtures Acetonitrile, mesitylene (Fluka AG, mole [1-3]. -

Acetonitrile Safe Storage and Handling Guide Table of Contents
Acetonitrile Safe Storage and Handling Guide Table of Contents Introduction 1.0 Physical, chemical and thermodynamic properties 2.0 Storage and transfer 3.0 Transport 4.0 Fire safety 5.0 Occupational safety and health 6.0 Emergency response and environmental protection 7.0 Section 1: Introduction This booklet was prepared for individuals who handle or • The vapors of Acetonitrile, if inhaled at certain concentra- may come in contact with Acetonitrile. It is a compilation of tions, can produce serious acute (short term) toxicity, practical, understandable information designed to guide the including loss of consciousness or death. The duration of reader in responsible handling of Acetonitrile, and to answer exposure is also a factor, as is the contact of Acetonitrile commonly raised questions about Acetonitrile safety. Before liquid or vapor with the skin. However, if administered handling, always consult a current Materials Safety Data effectively, commercially available antidotes can in most Sheet (MSDS), available from INEOS, for information on cases preclude serious harm. the chemical. For more complete or detailed information, call the following numbers in the U.S.: About the Company Emergency Notification Number 877-856-3682 With facilities located in Lima, Ohio and Port Lavaca, Non-Emergency Information Number 866-363-2454 Texas in the United States, and Teeside in the United Kingdom, INEOS is the world’s largest producer and Information contained in this booklet is not intended to marketer of Acetonitrile, a co-product of Acrylonitrile replace any legal requirements for Acetonitrile handling and primarily used as a solvent in the production of pharma- storage. Supplemental or more detailed specifications may be ceuticals, agricultural products and fine chemicals. -

Drying of Organic Solvents: Quantitative Evaluation of the Efficiency of Several Desiccants
pubs.acs.org/joc Drying of Organic Solvents: Quantitative Evaluation of the Efficiency of Several Desiccants D. Bradley G. Williams* and Michelle Lawton Research Centre for Synthesis and Catalysis, Department of Chemistry, University of Johannesburg, P.O. Box 524, Auckland Park 2006, South Africa [email protected] Received August 12, 2010 Various commonly used organic solvents were dried with several different drying agents. A glovebox- bound coulometric Karl Fischer apparatus with a two-compartment measuring cell was used to determine the efficiency of the drying process. Recommendations are made relating to optimum drying agents/conditions that can be used to rapidly and reliably generate solvents with low residual water content by means of commonly available materials found in most synthesis laboratories. The practical method provides for safer handling and drying of solvents than methods calling for the use of reactive metals, metal hydrides, or solvent distillation. Introduction facilities. Accordingly, users of the published drying methods rely upon procedures that generally have little or no quanti- Laboratories involved with synthesis require efficient methods fied basis of application and generate samples of unknown with which to dry organic solvents. Typically, prescribed water content. In a rather elegant exception, Burfield and co- methods1 are taken from the literature where little or no workers published a series of papers3 some three decades ago quantitative analysis accompanies the recommended drying in which the efficacy of several drying agents was investi- method. Frequently, such methods call for the use of highly gated making use of tritiated water-doped solvents. The reactive metals (such as sodium) or metal hydrides, which drying process was followed by scintillation readings, and increases the risk of fires or explosions in the laboratory. -
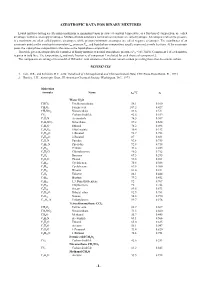
Azeotropic Data for Binary Mixtures
AZEOTROPIC DATA FOR BINARY MIXTURES Liquid mixtures having an extremum (maximum or minimum) vapor pressure at constant temperature, as a function of composition, are called azeotropic mixtures, or simply azeotropes. Mixtures that do not show a maximum or minimum are called zeotropic. Azeotropes in which the pressure is a maximum are often called positive azeotropes, while pressure-minimum azeotropes are called negative azeotropes. The coordinates of an azeotropic point are the azeotropic temperature taz, pressure Paz , and liquid-phase composition, usually expressed as mole fractions. At the azeotropic point, the vapor-phase composition is the same as the liquid-phase composition. This table gives azeotropic data for a number of binary mixtures at normal atmospheric pressure (Paz =101.3 kPa). Component 1 of each mixture is given in bold face. The temperature taz and mole fraction x1 of component 1 are listed for each choice of component 2. The components are arranged in a modified Hill order, with substances that do not contain carbon preceding those that do contain carbon. REFERENCES 1. Lide, D.R., and Kehiaian, H.V., CRC Handbook of Thermophysical and Thermochemical Data, CRC Press, Boca Raton, FL, 1994. 2. Horsley, L.H., Azeotropic Data, III, American Chemical Society, Washington, D.C., 1973. Molecular ° formula Name taz/ C x1 Water H2O CHCl3 Trichloromethane 56.1 0.160 CH2O2 Formic acid 107.2 0.427 CH3NO2 Nitromethane 83.6 0.511 CS2 Carbon disulfide 42.6 0.109 C2H3N Acetonitrile 76.5 0.307 C2H5NO2 Nitroethane 87.2 0.624 C2H6O Ethanol -

Intermolecular Interactions Involving Epoxy Polymer, Mixed Monolayers, and Polar Solvents Dmitri V
Published on Web 08/07/2002 Chemical Force Spectroscopy in Heterogeneous Systems: Intermolecular Interactions Involving Epoxy Polymer, Mixed Monolayers, and Polar Solvents Dmitri V. Vezenov,*,† Andrew V. Zhuk,‡ George M. Whitesides,† and Charles M. Lieber† Contribution from the Department of Chemistry and Chemical Biology and DiVision of Applied Sciences and Engineering, HarVard UniVersity, Cambridge, Massachusetts 02138 Received February 16, 2002 Abstract: We used chemical force microscopy (CFM) to study adhesive forces between surfaces of epoxy resin and self-assembled monolayers (SAMs) capable of hydrogen bonding to different extents. The influence of the liquid medium in which the experiments were carried out was also examined systematically. The molecular character of the tip, polymer, and liquid all influenced the adhesion. Complementary macroscopic contact angle measurements were used to assist in the quantitative interpretation of the CFM data. A direct correlation between surface free energy and adhesion forces was observed in mixed alcohol-water solvents. An increase in surface energy from 2 to 50 mJ/m2 resulted in an increase in adhesion from 4-8 nN to 150-300 nN for tips with radii of 50-150 nm. The interfacial surface energy for identical nonpolar surface groups of SAMs was found not to exceed 2 mJ/m2. An analysis of adhesion data suggests that the solvent was fully excluded from the zone of contact between functional groups on the tip and sample. With a nonpolar SAM, the force of adhesion increased monotonically in mixed solvents of higher water content; whereas, with a polar SAM (one having a hydrogen bonding component), higher water content led to decreased adhesion.