Interactions Between Phytoplankton Dynamics, Nutrient Loads and the Biogeochemistry of the Gippsland Lakes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
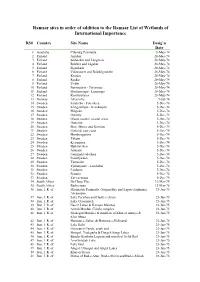
Ramsar Sites in Order of Addition to the Ramsar List of Wetlands of International Importance
Ramsar sites in order of addition to the Ramsar List of Wetlands of International Importance RS# Country Site Name Desig’n Date 1 Australia Cobourg Peninsula 8-May-74 2 Finland Aspskär 28-May-74 3 Finland Söderskär and Långören 28-May-74 4 Finland Björkör and Lågskär 28-May-74 5 Finland Signilskär 28-May-74 6 Finland Valassaaret and Björkögrunden 28-May-74 7 Finland Krunnit 28-May-74 8 Finland Ruskis 28-May-74 9 Finland Viikki 28-May-74 10 Finland Suomujärvi - Patvinsuo 28-May-74 11 Finland Martimoaapa - Lumiaapa 28-May-74 12 Finland Koitilaiskaira 28-May-74 13 Norway Åkersvika 9-Jul-74 14 Sweden Falsterbo - Foteviken 5-Dec-74 15 Sweden Klingavälsån - Krankesjön 5-Dec-74 16 Sweden Helgeån 5-Dec-74 17 Sweden Ottenby 5-Dec-74 18 Sweden Öland, eastern coastal areas 5-Dec-74 19 Sweden Getterön 5-Dec-74 20 Sweden Store Mosse and Kävsjön 5-Dec-74 21 Sweden Gotland, east coast 5-Dec-74 22 Sweden Hornborgasjön 5-Dec-74 23 Sweden Tåkern 5-Dec-74 24 Sweden Kvismaren 5-Dec-74 25 Sweden Hjälstaviken 5-Dec-74 26 Sweden Ånnsjön 5-Dec-74 27 Sweden Gammelstadsviken 5-Dec-74 28 Sweden Persöfjärden 5-Dec-74 29 Sweden Tärnasjön 5-Dec-74 30 Sweden Tjålmejaure - Laisdalen 5-Dec-74 31 Sweden Laidaure 5-Dec-74 32 Sweden Sjaunja 5-Dec-74 33 Sweden Tavvavuoma 5-Dec-74 34 South Africa De Hoop Vlei 12-Mar-75 35 South Africa Barberspan 12-Mar-75 36 Iran, I. R. -

Hudsonian Godwit (Limosa Haemastica) at Orielton Lagoon, Tasmania, 09/03/2018
Hudsonian Godwit (Limosa haemastica) at Orielton Lagoon, Tasmania, 09/03/2018 Peter Vaughan and Andrea Magnusson E: T: Introduction: This submission pertains to the observation and identification of a single Hudsonian Godwit (Limosa haemastica) at Orielton Lagoon, Tasmania, on the afternoon of the 9th of March 2018. The bird was observed in the company of 50 Bar-tailed Godwits (Limosa lapponica) and a single Black-tailed Godwit (Limosa limosa), and both comparative and diagnostic features were used to differentiate it from these species. Owing to the recognised difficulties in differentiating similar species of wading birds, this submission makes a detailed analysis of the features used to identify the godwit in question. If accepted, this sighting would represent the eighth confirmed record of this species in Australia, and the second record of this species in Tasmania. Sighting and Circumstances: The godwit in question was observed on the western side of Orielton Lagoon in Tasmania, at approximately 543440 E 5262330 N, GDA 94, 55G. This location is comprised of open tidal mudflats abutted by Sarcicornia sp. saltmarsh, and is a well- documented roosting and feeding site for Bar-tailed Godwits and several other species of wading bird. This has included, for the past three migration seasons, a single Black-tailed Godwit (extralimital at this location) associating with the Bar-tailed Godwit flock. The Hudsonian Godwit was observed for approximately 37 minutes, between approximately 16:12 and 16:49, although the mixed flock of Bar-tailed Godwits and Black-tailed Godwits was observed for 55 minutes prior to this time. The weather during observation was clear skies (1/8 cloud cover), with a light north- easterly breeze and relatively warm temperatures (wind speed nor exact temperature recorded). -

Pitt Water – Orielton Lagoon Tasmania Ecological Character
Pitt WaterWater – Orielton Lagoon Tasmania Ecological Character Description AugustDraft 1 2012August 2009 Pitt Water – Orielton Lagoon Tasmania Ecological Character Description August 2012 Introductory Notes The Environment Protection and Biodiversity Conservation Act 1999 (EPBC Act) prohibits actions that are likely to have a significant impact on the ecological character of a Ramsar wetland unless the Commonwealth Environment Minister has approved the taking of the action, or some other provision in the EPBC Act allows the action to be taken. The information in this ECD Publication does not indicate any commitment to a particular course of action, policy position or decision. Further, it does not provide assessment of any particular action within the meaning of the Environment Protection and Biodiversity Conservation Act 1999 (Cth), nor replace the role of the Minister or his delegate in making an informed decision to approve an action. The Water Act 2007 requires that in preparing the [Murray-Darling] Basin Plan, the Murray Darling Basin Authority (MDBA) must take into account Ecological Character Descriptions of declared Ramsar wetlands prepared in accordance with the National Framework. This ECD publication is provided without prejudice to any final decision by the Administrative Authority for Ramsar in Australia on change in ecological character in accordance with the requirements of Article 3.2 of the Ramsar Convention. Disclaimer While reasonable efforts have been made to ensure the contents of this ECD are correct, the Commonwealth of Australia as represented by the Department of Sustainability, Environment, Water, Population and Communities does not guarantee and accepts no legal liability whatsoever arising from or connected to the currency, accuracy, completeness, reliability or suitability of the information in this ECD. -

CERCA Baseline Report Final
FINAL REPORT TO NHT COASTAL & ESTUARINE RESOURCE CONDITION ASSESSMENT A BASELINE SURVEY IN THE SOUTHERN NRM REGION, TASMANIA Nepelle Temby and Christine Crawford June 2008 Coastal and Estuarine Resource Condition Assessment: A baseline survey in the Southern NRM Region, Tasmania Disclaimer The content of this report has been based on existing information that will be subject to change as new information becomes available. Every effort has been made to ensure that the information contained in this strategy is accurate. Further information is available from the references listed. The opinions expressed in this report are those of the author/s and are not necessarily those of the Tasmanian Aquaculture and Fisheries Institute. ©Tasmanian Aquaculture and Fisheries Institute, University of Tasmania 2008. Marine Research Laboratories – Tasmanian Aquaculture and Fisheries Institute, University of Tasmania, Private Bag 49, Hobart, Tasmania, 7001. Email: [email protected] Ph: (03) 6227 7224 Fax: (03) 6227 8035 Contents SUMMARY ...................................................................................................................................................................1 ACKNOWLEDGEMENTS ............................................................................................................................................2 ACRONYMS AND ABBREVIATIONS .........................................................................................................................3 INTRODUCTION .........................................................................................................................4 -

King Island News for 8 Days Over Easter This Year
YELLOW THROAT The newsletter of BirdLife Tasmania a branch of BirdLife Australia Number 69, May 2013 Bob Brown at General Meeting Greg Irons to speak in July Life Sciences Building, University of Greg became the director of the Bonorong Wildlife Tasmania, Thursday, 9 May, 8.00 p.m. Sanctuary at just 25 years of age, and has so far enlisted more than 800 volunteers. The rescue service runs 24 Dr Bob Brown, founder and former leader of the Greens hours a day, seven days a week, and in the last 12 months Party, former senator for Tasmania, and current director of has taken over 5000 calls. As a private sanctuary that cares the Australian chapter of Sea Shepherd, will be the speaker for orphaned and injured wildlife, Bonorong depends on at the May General Meeting. Dr Brown will be speaking donations and visitor entries. about the impact that birds have had in his life. Greg is also a familiar face in the children’s ward of the Royal Hobart Hospital, visiting with baby wombats and Meeting venue: Life Sciences Lecture Theatre 1, Life blue-tongue lizards to brighten sick children’s days and Sciences Building, University of Tasmania, Sandy Bay. help spread his conservation message. Greg’s deep under- Access and parking are from College Road or from the standing of Tasmania’s wildlife, and his passion for parking area outside the University Centre via the conservation values, is helping preserve the state’s pedestrian bridge over Churchill Avenue. precious environment. Bonorong Wildlife Sanctuary BYO Barbecue for BirdLife Tasmania, 18 May Bonorong Wildlife Sanctuary is hosting a BYO barbecue for BirdLife Tasmania members on Saturday 18 May at 5.00 p.m. -

Pembroke Park Master Plan
PEMBROKE PARK MASTER PLAN PREPARED FOR SORELL COUNCIL FEBRUARY 2015 PEMBROKE PARK MASTER PLAN prepared for Sorell Council Inspiring Place Pty Ltd Placemaking: Environmental Planning, Landscape Architecture, Tourism + Recreation 210 Collins St Hobart TAS 7000 T: 03 6231-1818 E: [email protected] ACN 58 684 792 133 Date Version 28.11.14 Draft Master Plan to Council 23.02.15 Revised Master Plan following Council review CONTENTS Section 1 Introduction ..............................................................................................................1 1.1 Background .............................................................................................................1 1.2 Preparation of the Master Plan ...............................................................................2 Section 2 Context......................................................................................................................5 2.1 Policy Framework....................................................................................................5 2.2 Trends .....................................................................................................................8 2.2.1 Population Trends ...................................................................................8 2.2.2 Sport and Recreation Participation Trends .............................................10 2.3 Current Facilities and Use.......................................................................................14 2.4 Club/Group Issues and Opportunities -

Appendix 7-2 Protected Matters Search Tool (PMST) Report for the Risk EMBA
Environment plan Appendix 7-2 Protected matters search tool (PMST) report for the Risk EMBA Stromlo-1 exploration drilling program Equinor Australia B.V. Level 15 123 St Georges Terrace PERTH WA 6000 Australia February 2019 www.equinor.com.au EPBC Act Protected Matters Report This report provides general guidance on matters of national environmental significance and other matters protected by the EPBC Act in the area you have selected. Information on the coverage of this report and qualifications on data supporting this report are contained in the caveat at the end of the report. Information is available about Environment Assessments and the EPBC Act including significance guidelines, forms and application process details. Report created: 13/09/18 14:02:20 Summary Details Matters of NES Other Matters Protected by the EPBC Act Extra Information Caveat Acknowledgements This map may contain data which are ©Commonwealth of Australia (Geoscience Australia), ©PSMA 2010 Coordinates Buffer: 1.0Km Summary Matters of National Environmental Significance This part of the report summarises the matters of national environmental significance that may occur in, or may relate to, the area you nominated. Further information is available in the detail part of the report, which can be accessed by scrolling or following the links below. If you are proposing to undertake an activity that may have a significant impact on one or more matters of national environmental significance then you should consider the Administrative Guidelines on Significance. World Heritage Properties: 11 National Heritage Places: 13 Wetlands of International Importance: 13 Great Barrier Reef Marine Park: None Commonwealth Marine Area: 2 Listed Threatened Ecological Communities: 14 Listed Threatened Species: 311 Listed Migratory Species: 97 Other Matters Protected by the EPBC Act This part of the report summarises other matters protected under the Act that may relate to the area you nominated. -

Pitt Water – Orielton Lagoon Tasmania Ecological Character Description Augustdraft 1 August2012 2009
Pitt Water – Orielton Lagoon Tasmania Ecological Character Description AugustDraft 1 August2012 2009 Pitt Water – Orielton Lagoon Tasmania Ecological Character Description August 2012 Introductory Notes The Environment Protection and Biodiversity Conservation Act 1999 (EPBC Act) prohibits actions that are likely to have a significant impact on the ecological character of a Ramsar wetland unless the Commonwealth Environment Minister has approved the taking of the action, or some other provision in the EPBC Act allows the action to be taken. The information in this ECD Publication does not indicate any commitment to a particular course of action, policy position or decision. Further, it does not provide assessment of any particular action within the meaning of the Environment Protection and Biodiversity Conservation Act 1999 (Cth), nor replace the role of the Minister or his delegate in making an informed decision to approve an action. The Water Act 2007 requires that in preparing the [Murray-Darling] Basin Plan, the Murray Darling Basin Authority (MDBA) must take into account Ecological Character Descriptions of declared Ramsar wetlands prepared in accordance with the National Framework. This ECD publication is provided without prejudice to any final decision by the Administrative Authority for Ramsar in Australia on change in ecological character in accordance with the requirements of Article 3.2 of the Ramsar Convention. Disclaimer While reasonable efforts have been made to ensure the contents of this ECD are correct, the Commonwealth of Australia as represented by the Department of Sustainability, Environment, Water, Population and Communities does not guarantee and accepts no legal liability whatsoever arising from or connected to the currency, accuracy, completeness, reliability or suitability of the information in this ECD. -

Ramsar National Report to COP13
Ramsar National Report to COP13 Section 1: Institutional Information Important note: the responses below will be considered by the Ramsar Secretariat as the definitive list of your focal points, and will be used to update the information it holds. The Secretariat’s current information about your focal points is available at http://www.ramsar.org/search-contact. Name of Contracting Party The completed National Report must be accompanied by a letter in the name of the Head of Administrative Authority, confirming that this is the Contracting Party’s official submission of its COP13 National Report. It can be attached to this question using the "Manage documents" function (blue symbol below) › Australia You have attached the following documents to this answer. Ramsar_National_Report-Australia-letter_re_submission-signed-Jan_2018.docx.pdf - Letter from Head of Australia's Ramsar Administrative Authority Designated Ramsar Administrative Authority Name of Administrative Authority › Commonwealth Environmental Water Office Australian Government Department of the Environment and Energy Head of Administrative Authority - name and title › Mr Mark TaylorAssistant Secretary, Wetlands, Policy and Northern Water UseCommonwealth Environmental Water Office Mailing address › GPO Box 787 Canberra ACT 2601 Australia Telephone/Fax › +61 2 6274 1904 Email › [email protected] Designated National Focal Point for Ramsar Convention Matters Name and title › Ms Leanne WilkinsonAssistant Director, Wetlands Section Mailing address › Wetlands, Policy and -

Information Sheet on Ramsar Wetlands (RIS) – 2009-2014 Version
Information Sheet on Ramsar Wetlands (RIS) – 2009-2014 version Available for download from http://www.ramsar.org/doc/ris/key_ris_e.doc and http://www.ramsar.org/pdf/ris/key_ris_e.pdf Categories approved by Recommendation 4.7 (1990), as amended by Resolution VIII.13 of the 8th Conference of the Contracting Parties (2002) and Resolutions IX.1 Annex B, IX.6, IX.21 and IX. 22 of the 9th Conference of the Contracting Parties (2005). Notes for compilers: 1. The RIS should be completed in accordance with the attached Explanatory Notes and Guidelines for completing the Information Sheet on Ramsar Wetlands. Compilers are strongly advised to read this guidance before filling in the RIS. 2. Further information and guidance in support of Ramsar site designations are provided in the Strategic Framework and guidelines for the future development of the List of Wetlands of International Importance (Ramsar Wise Use Handbook 17, 4th edition). 3. Once completed, the RIS (and accompanying map(s)) should be submitted to the Ramsar Secretariat. Compilers should provide an electronic (MS Word) copy of the RIS and, where possible, digital copies of all maps. 1. Name and address of the compiler of this form: FOR OFFICE USE ONLY. Department of Primary Industries, Parks, Water and DD MM YY Environment (DPIPWE) GPO Box 44 HOBART Tasmania 7001 Australia Designation date Site Reference Number +61 3 6165 4396 [email protected] 2. Date this sheet was completed/updated: May 2014 3. Country: Australia 4. Name of the Ramsar site: The precise name of the designated site in one of the three official languages (English, French or Spanish) of the Convention. -

Australia's Ramsar Wetlands Used by Shorebirds
Australia’s Ramsar wetlands used by shorebirds Listed below are Ramsar wetlands that are significant shorebird habitats with some of the migratory and resident species found at that location. If a more detailed species list for a particular Ramsar wetland is required, please email [email protected] State Wetland Shorebird species Northern Cobourg Peninsular Migratory species of the East Asian Australasian Flyway (EAAF) Territory Kakadu National Park Australian Pratincole, Comb-crested Jacana, Curlews, Sandpipers Queensland Bowling Green Bay Australian Pratincole, Curlews, Dunlin, Godwits, Knots, Latham's Snipe, Red-necked Stint, Ruddy Turnstone, Ruff, Sanderling, Sandpipers, Whimbrel Currawinya Lakes Black-winged Stilt, Common Greenshank, Godwits, Plovers, Red-necked Avocet, Ruddy Turnstone, Sandpipers, Snipes, Stints, important inland migration route for EAAF species Shoalwater and Corio Bay areas Bar-tailed Godwit, Beach Stone-curlew, Curlews, Great Knot, Pied Oystercatcher, Tattlers, Terek Sandpiper, Whimbrel Great Sandy Strait (Tin Can Bay/Inlet) 38 shorebird species including: Bar-tailed Godwit, Common Greenshank, Curlews, Grey Plover, Grey-tailed Tattler, Lesser Sand Plover, Pied Oystercatcher, Terek Sandpiper, Whimbrel Moreton Bay 43 shorebird species including: Bar-tailed Godwit, Beach Stone-curlew, Curlews, Grey-tailed Tattler, Knots, Pied Oystercatcher, Plovers, Red-necked Stint, Sharp-tailed Sandpiper, Whimbrel New South Myall lakes Bar-tailed Godwit, Black-winged Stilt, Bush Stone-curlew, Common Greenshank, Eastern -
Ramsar "Wetlands of International Importance" with Lake Habitats
Ramsar "Wetlands of International Importance" with lake habitats The Ramsar Convention is an intergovernmental treaty adopted in 1971 which relates to "all areas where water is the primary factor controlling the environment and associated plant and animal life", thus including lakes. Wetlands are listed as "wetlands of international importance" after being nominated by their resident country and meeting at least one of eight criteria set out under the Ramsar Convention. Over 300 Ramsar sites are primarily lake habitats, and another 350 include at least some lake habitat. The chart below is based on Ramsar data obtained from Wetlands International, Oct. 2002. Ramsar sites in bold indicate that the site is primarily lake habitat; Ramsar sites not appearing in bold are sites at which some lake habitat occurs, but it is not the main habitat of the site. Country Ramsar site Lake habitat type Algeria Chott El Hodna Intermittent saline Algeria Lac des Oiseaux, ou Garaet et Touyour Permanent fresh Algeria Lac Oubeïra Permanent fresh Algeria Lac Tonga Permanent fresh Algeria Sebkha d'Oran Intermittent saline Algeria La Vallée d'Iherir Intermittent fresh Algeria Chott Merrouane et Oued Khrouf Permanent saline Algeria Marais de la Macta Permanent saline Algeria Oasis de Tamantit et Sid Ahmed Timmi Intermittent saline Algeria Oasis de Ouled Saïd Intermittent saline Argentina Laguna Blanca Permanent fresh Argentina Lagunas y Esteros del Iberá Permanent fresh Argentina Lagunas de Guanacache Intermittent fresh Argentina Bañados del Río Dulce y Laguna