A New Flavonol Glycoside from Kalanchoe Pinnata Leaves
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
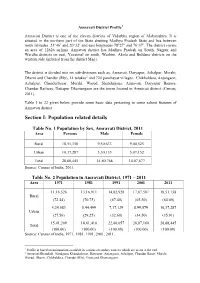
Section I: Population Related Details
Amravati District Profile1 Amravati District is one of the eleven districts of Vidarbha region of Maharashtra. It is situated in the northern part of the State abutting Madhya Pradesh State and lies between north latitudes 21°46’ and 20°32’ and east longitudes 78°27’ and 76°37’. The district covers an area of 12626 sq.kms. Amravati district has Madhya Pradesh on South, Nagpur and Wardha districts on east, Yavatmal on south, Washim, Akola and Buldana districts on the western side (inferred from the district Map). The district is divided into six sub-divisions such as, Amravati, Daryapur, Achalpur, Morshi, Dharni and Chandur (Rly), 14 talukas2 and 724 panchayat villages. Chikhaldara, Anjangaon, Achalpur, Chandurbazar, Morshi, Warud, Shendurjana, Amravati, Daryapur Banosa, Chandur Railway, Dattapur Dhamangaon are the towns located in Amravati district (Census, 2011). Table 1 to 22 given below provide some basic data pertaining to some salient features of Amravati district. Section I: Population related details Table No. 1 Population by Sex, Amravati District, 2011 Area Persons Male Female Rural 18,51,158 9,50,633 9,00,525 Urban 10,37,287 5,30,135 5,07,152 Total 28,88,445 14,80,768 14,07,677 Source: Census of India, 2011. Table No. 2 Population in Amravati District, 1971 – 2011 Area 1971 1981 1991 2001 2011 11,16,526 13,16,911 14,82,928 17,07,581 18,51,158 Rural (72.44) (70.75) (67.40) (65.50) (64.09) 4,24,683 5,44,499 7,17,129 8,99,579 10,37,287 Urban (27.56) (29.25) (32.60) (34.50) (35.91) 15,41,209 18,61,410 22,00,057 26,07,160 28,88,445 Total (100.00) (100.00) (100.00) (100.00) (100.00) Source: Census of India, 1971, 1981, 1991, 2001, 2011. -

Impact of Global Warming on Rainfall and Wheat Production of Amravati District in Vidarbha, India
Internationl Research Journal of Agricultural Economics and Statistics Volume 4 | Issue 1 | March, 2013 | 18-24 Research Paper Impact of global warming on rainfall and wheat production of Amravati district in Vidarbha, India D.T. DESHMUKH AND H.S. LUNGE See end of the paper for ABSTRACT : India is an agricultural country and agriculture production very much depends on temperature and authors’ affiliations rainfall. Mostly agriculture in India is rainfed. Vidarbha is the eastern region of Maharashtra State. Nearly 89 per Correspondence to : cent of cultivated area of Vidarbha is under rain fed farming.Now a days global warming has become a great D.T. DESHMUKH challenge for the agrarian economy of India. This paper analyses the agriculture production of wheat, average Department of Statistics, maximum and minimum temperatures and total rainfall data for eighteen years obtained from IMD, Pune for Brijlal Biyani Science Amravati district of Vidarbha. Regression and correlation analysis is obtained and their significance is tested. It College, AMRAVATI (M.S.) INDIA is observed that minimum temperature is increasing significantly for Amravati district where as rainfall and wheat Email : dtdeshmukh.1721 production revealed decreasing trend. Increased temperature and reduced rainfall affects wheat production in @gmail.com Amravati district. KEY WORDS : Agriculture, Climate Variables, Correlation, Regression, t-test Paper History : Received : 26.07.2012; HOW TO CITE THIS PAPER : Deshmukh, D.T. and Lunge, H.S. (2013). Impact of global warming on rainfall and wheat Revised : 12.01.2013; production of Amravati district in Vidarbha, India, Internat. Res. J. agric. Eco. & Stat., 4 (1) : 18-24. Accepted: 14.02.2013 INTRODUCTION In India, monsoons are getting more variable, less predictable and very extreme. -

University Grants Commission Socio and Economic Study and Critical Analysis of the Causes and Factors Leading to the Suicides Of
University Grants Commission (W.R.O) Pune SUMMARY - Minor Research Project - * Subject * Socio And Economic Study and critical analysis of the Causes and Factors Leading to the Suicides of Farmers in Amravati District Name of Teacher – Kumar Bobade. File NO. 23 – 007/6 (WRO) Dated 03/02/07. Amount Sanction Rs. 35000/- Shri Shivaji College, Amravati. MINOR REASERCH PROJECT Summary “Socio And Economic Study and Critical analysis of the Causes and Factors Leading to the Suicides of Farmers in Amravati District” Chapter I - Introduction India is agriculture based country. Near about 80% population means more than 2/3 nation belongs to villages. Agriculture and agriculture based industries are only meant for bread and butter for these people. It is a well-known fact that agriculture is the backbone of economy of this country. Father of nation’s Mahatma Gandhi was well familiar with this fact and that is why agriculture and related industries were emphasized by him. The Government since independence has given the importance to the agriculture and agriculture based industries and various agriculture development schemes have been launched. In five year development plan Agriculture is the prominent factor. Green revolution was planned and succeeded to develop agriculture and self- independence in food grains to make it prosper and self-independent. It was the beginning for adopting new technologies, new methods and inventions in agriculture. Agriculture universities were established. But it is a question that did they fulfill the needs and useful betterment of farmers? Can we say that the farmers in this country are self-developed, mentally capable and economically stabled? Overall answer is Negative. -

Sr No Age Sex Address
RTPCR SR NO AGE SEX ADDRESS 1 60 M DHAMTRI 2 51 M TIOSA Femal 3 25 SALORA e Femal 4 60 SATARGAON e Femal 5 32 TIOSA e Femal 6 42 SHENDOLA e 7 13 M SHENDOLA 8 23 M SURWADI KH 9 24 M TALEGAON TH 10 38 M MOZARI 11 36 M MAMADAPUR 12 24 M TIOSA 13 48 M ANKWADI Femal 14 40 SATARGAON e Femal 15 29 SURWADI KH e 16 5 M SATARGAON Femal 17 8 TIOSA e 18 20 M TIOSA 19 30 M MOZARI Femal 20 38 KURHA e 21 77 M PARDI 22 28 M TIOSA 23 30 M MOZARI 24 24 M TAKRKHEDA Femal 25 22 TIOSA e 26 52 M SHIVANGAON 27 29 M Narayan Nager Daryapur 28 21 M Sai Nager daryapur 29 9 M DAHISATH AMT Femal 30 28 Panjabrao Colony Daryapur e 31 66 M Sainik Colony Daryapur 32 29 M Sainik Colony Daryapur Sahajeevan Colony 33 12 M Daryapur Femal 34 27 Sainagar Daryapur e 35 58 M Ramabai Nager Daryapur 36 53 M Mandwa Chandur Rly 37 17 M Bank Colony Chandur Rly 38 32 M Lalkhed Chandur Rly 39 32 M Shivaji Nagar Chandur Rly 40 53 M Satefal Chandur Rly Patrikar colony Chandur 41 41 M Rly 42 42 M Virul Ronghe Chandur Rly Femal 43 43 Rajura Chandur Rly e Femal 44 42 Satefal Chandur Rly e 45 2 M Satefal Chandur Rly 46 49 M Shivaji Nagar Chandur Rly Femal 47 60 Kalamgaon Chandur Rly e 48 40 M Kalamgaon Chandur Rly 49 61 M Kalamgaon Chandur Rly Femal 50 68 Kalamgaon Chandur Rly e 51 38 M Depo Chandur Rly Femal 52 60 Lalkhed Chandur Rly e Femal 53 8 Kalamgaon Chandur Rly e 54 44 M Kranti Chowk Chandur Rly Femal 55 4 Kalamgaon Chandur Rly e Sawanga Vithoba Chandur 56 40 M Rly Sawanga Vithoba Chandur 57 66 M Rly Sawanga Vithoba Chandur 58 70 M Rly Femal 59 35 Malipura Morshi e 60 47 M Pala Morshi -

Reg. No Name in Full Residential Address Gender Contact No. Email Id Remarks 9421864344 022 25401313 / 9869262391 Bhaveshwarikar
Reg. No Name in Full Residential Address Gender Contact No. Email id Remarks 10001 SALPHALE VITTHAL AT POST UMARI (MOTHI) TAL.DIST- Male DEFAULTER SHANKARRAO AKOLA NAME REMOVED 444302 AKOLA MAHARASHTRA 10002 JAGGI RAMANJIT KAUR J.S.JAGGI, GOVIND NAGAR, Male DEFAULTER JASWANT SINGH RAJAPETH, NAME REMOVED AMRAVATI MAHARASHTRA 10003 BAVISKAR DILIP VITHALRAO PLOT NO.2-B, SHIVNAGAR, Male DEFAULTER NR.SHARDA CHOWK, BVS STOP, NAME REMOVED SANGAM TALKIES, NAGPUR MAHARASHTRA 10004 SOMANI VINODKUMAR MAIN ROAD, MANWATH Male 9421864344 RENEWAL UP TO 2018 GOPIKISHAN 431505 PARBHANI Maharashtra 10005 KARMALKAR BHAVESHVARI 11, BHARAT SADAN, 2 ND FLOOR, Female 022 25401313 / bhaveshwarikarmalka@gma NOT RENEW RAVINDRA S.V.ROAD, NAUPADA, THANE 9869262391 il.com (WEST) 400602 THANE Maharashtra 10006 NIRMALKAR DEVENDRA AT- MAREGAON, PO / TA- Male 9423652964 RENEWAL UP TO 2018 VIRUPAKSH MAREGAON, 445303 YAVATMAL Maharashtra 10007 PATIL PREMCHANDRA PATIPURA, WARD NO.18, Male DEFAULTER BHALCHANDRA NAME REMOVED 445001 YAVATMAL MAHARASHTRA 10008 KHAN ALIMKHAN SUJATKHAN AT-PO- LADKHED TA- DARWHA Male 9763175228 NOT RENEW 445208 YAVATMAL Maharashtra 10009 DHANGAWHAL PLINTH HOUSE, 4/A, DHARTI Male 9422288171 RENEWAL UP TO 05/06/2018 SUBHASHKUMAR KHANDU COLONY, NR.G.T.P.STOP, DEOPUR AGRA RD. 424005 DHULE Maharashtra 10010 PATIL SURENDRANATH A/P - PALE KHO. TAL - KALWAN Male 02592 248013 / NOT RENEW DHARMARAJ 9423481207 NASIK Maharashtra 10011 DHANGE PARVEZ ABBAS GREEN ACE RESIDENCY, FLT NO Male 9890207717 RENEWAL UP TO 05/06/2018 402, PLOT NO 73/3, 74/3 SEC- 27, SEAWOODS, -

A Detailed District Survey Report of Amravati
DSR- AMRAVATI A Detailed District Survey Report of Amravati Collector office; Amravati. 1 DSR- AMRAVATI Index Preface 1. Introduction 2. Overview of mining activity in the district 3. List of the Mining Leases in the district 4. Details of Royalty or Revenue received in last three years 5. Details of Production of sand or minor mineral in last three years 6. Deposition of sediments in the rivers of the district 7. General profile of the district 8. Land utilization pattern in the district 9. Physiography of the district 10. Rainfall 11. Geology and Mineral wealth 12. Major and Minor Rivers of the District 13. General recommendation 2 DSR- AMRAVATI Preface In Compliance to the Notification Issued by the Ministry Of Environment, Forest and Climate change Dated 15.01.2016, the preparation of District survey report of River bed mining and other minor minerals is in accordance appendix 10 of the notification. It is also mentioned here that the procedure of preparation of District Survey Report is as per notification guidelines. Every efforts have been made to cover sand mining locations, area & overview of mining activity in the district with all its relevant features pertaining to geology & mineral wealth in replenish able and non- replenish able areas of rivers, stream and other sand sources. This report will be a model and guiding document which is a compendium of available mineral resources, geographical set up, environmental and ecological set up of the district and is based on data of various departments, published reports, and websites. The data may vary due to floods, heavy rains and other natural calamities. -

District Survey Report for Sand Mining Or River Bed Mining
DRAFT DSR REPORT DISTRICT SURVEY REPORT FOR SAND MINING OR RIVER BED MINING Prepared Under A] Appendix –X Of MoEFCC, GoI. Notification S.O. 141(E) Dated 15.1.2016 B] Sustainable Sand Mining Guidelines C] MoEFCC, GoI. Notification S.O. 3611(E) Dated 25.07.2018 AMRAVATI - 2019-20 MAHARASHTRA PREFACE The Ministry of Environment, Forests & Climate Change (MoEF&CC), Government of India, made Environmental Clearance (EC) for mining of minerals mandatory through its Notification of 27th January, 1994 under the provisions of Environment Protection Act, 1986. Keeping in view the experience gained in environmental clearance process over a period of one decade, the MoEF&CC came out with Environmental Impact Notification, SO 1533 (E), dated 14th September 2006. Now again Ministry of Environment, Forests & Climate Change (MoEF&CC), Government of India amended the notification S.O. th 141(E) Dated 15TH January, 2016 vide S.O. 3611(E) Dated 25 July, 2018. It has been made mandatory to obtain environmental clearance for different kinds of development projects as listed in Appendix-X of the Notification. Further, In pursuance to the order of Hon’ble Supreme Court dated the 27th February, 2012 in I.A. No.12- 13 of 2011 in Special Leave Petition (C) No.19628-19629 of 2009, in the matter of Deepak Kumar etc. Vs. State of Haryana and Others etc., prior environmental clearance has now become mandatory for mining of minor minerals irrespective of the area of mining lease; And also in view of the Hon’ble National Green Tribunal, order dated the 13TH January, -

Rejected-Application-List.Pdf
SANT GADGE BABA AMRAVATI UNIVERSITY LIST OF REJECTED APPLICATION FORMS RECEIVED FOR REGISTRATION OF REGISTERD GRADUATES OF SANT GADGE BABA AMRAVATI UNIVERSITYUNDER SECTION 99 OF MAHARASHTRA UNIVERSITIES ACT,1994 & SECTION 131OF MAHARASHTRA PUBLIC UNIVERSITIES ACT,2016 FACULTY OF ARTS (INCLUDING FINE ART) Sr.No. Appl. No. Name & Address Lot No. Reason for Rejection AAFREEN QUDSIYA MEHMOOD Application form not signed 1 18850 01R09 AKOT FILE,AKOLA,AKOLA,AKOLA.444001 AAHOTE VINOD MAHADEO Attached PG Degree 2 43160 01OFR SHAMSHERPURA,JAMBHA BK,AKOLA. ABDUL GALIB ABDUL SATTAR Degree certificate not attached, 3 19151 01R01 FLT NO.A1 SAHARA APPARTMENT RPTS RD.AGAR BES OLD Prescribed application fee not deposited CITY AKOLA,AKOLA,AKOLA.444001 ABDUL RASHID ABDUL RAZZAK ABDUL Application form not signed 4 29853 01R44 TAJNAPETH AKOLA,,. ABDUL SAIFUDDNZAHEER TAJI Attached PG Degree 5 20389 01R06 BILAL NAGAR DIWANPURA WARD NO 03 MANGRULPIR,MANGRULPIR,WASHIM.44443 ABDUL SALIM SIDDIQUI Application form not signed 6 20514 01R06 AMRAVATI ,AMRAVATI,AMRAVATI.444604 ABHYANKAR WASUDEO SUKHDEORAO Attached PG Degree 7 15382 01R09 PANCHSHIL HOUSING SOCIETY CONGRESS NAGAR AMRAVATI,AMRAVATI,AMRAVATI.444606 ADASAD AARTI ARUNRAO Attached PG Degree 8 21238 01R07 C/O VIRENDRA BHOYAR, PRABHAT COLONY, NEAR ASMITA VIDYALAY, SHILANGAN ROAD, AMRAVATI,AMRAVATI,AMRAVATI. ADE SHUBHAM NIRANJAN Duplicate Form 9 11551 Du01 C/O MANISH GAJANANRAO PATIL, PANCHAWATI COLONY, VMV ROAD, AMRAVATI,AMRAVATI,AMRAVATI.444604 ADGOKAR MANGESH RAMESHWARRAO Residential proof not attached 10 33230 01R43 FLAT NO. 204, SHIROMANI APARTMENT, KONARK COLONY, B/H RANGOLI LAWN, KATHORA ROAD, AMRAVATI ,,. ADHAO ARCHANA GULABRAO Duplicate Form 11 18808 DU03 C/O HARSHAL SHRIKRISHNA MANKAR BEHIND BHARAT COMPUTER ASRA COLONY AKOT,AKOT,AKOLA. -

National Legal Services Authority
NATIONAL LEGAL SERVICES AUTHORITY DIRECTORY OF LEGAL SERVICES INSTITUTIONS 12/11, Jam Nagar House, Shahjahan Road, New Delhi-110 011, www.nalsa.gov.in e-mail: [email protected] Off: 23385321, Fax: 23382121 INDEX S.No. States Page No. (s) 1 Andhra Pradesh 1 – 9 2 Arunachal Pradesh 10-12 3 Assam 13-15 4 Bihar 16-19 5 Chhattisgarh 20-25 6 Goa 26-27 7 Gujarat 28-46 8 Haryana 47-50 9 Himachal Pradesh 51-57 10 J & K 58-62 11 Jharkhand 63-65 12 Karnataka 66-85 13 Kerala 86-93 14 Madhya Pradesh 94-112 15 Maharashtra 113-136 16 Manipur 137-138 17 Meghalaya 139-140 18 Mizoram 141-142 19 Nagaland 143-144 20 Orissa 145-156 21 Punjab 157-160 22 Rajasthan 161-179 23 Sikkim 180-181 24 Tamil Nadu 182-203 25 Telangana 204-212 26 Tripura 213-215 27 Uttar Pradesh 216-219 28 Uttarakhand 220-223 29 West Bengal 224-230 30 Andaman & Nicobar 231-232 31 UT Chandigarh 233 32 Dadar & Nagar Haveli 234 33 Daman & Diu 234 34 Delhi 235-236 35 Lakshadweep 237 36 Puducherry 238 1 ANDHRA PRADESH STATE LEGAL SERVICES AUTHORITY State Legal Services Office address and Front Office Telephone Email Authority (SLSA) telephone numbers No./ Helpline No. Andhra Pradesh State Legal Ground Floor, Interim 0863 2372760 apslsauthority@yahoo. Services Authority Judicial Complex,High com Court of A.P. Nelapadu, Amaravati, Guntur District. 0863 2372758 0863 2372759 High Court Legal Services Office address and Front Office Telephone Email Committee (HCLSC) telephone numbers No./ Helpline No. -

The History of Amravati the Ancient Name of Amravati Is "Udumbravati", Prakrut Form of This Is "Umbravati" and "Amravati" Is Known for Many Centuries with This Name
The History Of Amravati The ancient name of Amravati is "Udumbravati", prakrut form of this is "Umbravati" and "Amravati" is known for many centuries with this name. The mispronunciation form of this is Amravati and now the Amravati is known with the same. It is said that Amravati is named for its ancient Ambadevi temple. The ancient proof of existence of Amravati can get from stone carved inscription on the base of marble statue of God Adinath (Jain God) Rhishabhnath. This shows that, these statues were set up here in 1097. Govind Maha Prabhu visited Amravati in 13 th century, at the same time Warhad was under the rule of Deogiri's Hindu King (Yadav). In 14th century, there was famine (drought) in Amravati & people abandoned Amravati and left for Gujrat and Malva. The local people were returned back after several years at Amravati, the result of this was scanty population. In 16 th century, Mager Aurangpura (today's, 'Sabanpura') was presented for Jumma Majseed by Badashah Aurangjeb. This reveals that Muslims and Hindus lived together here. In 1722, Chhatrapati Shahoo Maharaj presented Amravati and Badnera to Shri Ranoji Bhosle, by the time Amravati was known as Bhosle ki Amravati. The city was reconstructed and prospered by Ranoji Bhosle after the treaty of Devgaon and Anjangaon Surji and victory over Gavilgad (Fort of Chikhaldara). The British general author Wellesly camped in Amravati, particular place is still recognized as camp, by Amravati people. The Amravati city came in to existence at the end of 18th century. Union state of Nijam and Bosale ruled the Amravati. -

Directorate of Vocational Education and Training, Maharashtra State 3, Mahapalika Marg, Post Box No
Government of Maharashtra Directorate of Vocational Education and Training, Maharashtra State 3, Mahapalika Marg, Post Box No. 10036, Mumbai 400 001 List of Hostels at Government Industrial Training Institute - शासकीय औोिगक िशण संथेतील वसतीगृहांची यादी Hostel Capacity S.N. Name of ITI City/_Village Taluka District Region Girls Boys Total 1 GOVT ITI, AKOLA, TAL: AKOLA, DIST: AKOLA AKOLA (M CORP.) AKOLA AKOLA AMRAVATI 0 300 300 GOVT ITI, ACHALPUR, TAL: ACHALPUR, DIST: 2 ACHALPUR (M CL) ACHALPUR AMRAVATI AMRAVATI 0 50 50 AMRAVATI GOVT ITI, AMRAVATI, TAL: AMRAVATI, DIST: 3 AMRAVATI (M CORP.) AMRAVATI AMRAVATI AMRAVATI 0 200 200 AMRAVATI GOVT ITI, (SCP), RAHATGAON, TAL: AMRAVATI, RAHATGAON AMRAVATI (M 4 AMRAVATI AMRAVATI AMRAVATI 50 50 100 DIST: AMRAVATI CORP.) GOVT ITI, (WOMAN), AMRAVATI, TAL: 5 AMRAVATI (M CORP.) AMRAVATI AMRAVATI AMRAVATI 100 0 100 AMRAVATI, DIST: AMRAVATI GOVT ITI, CHANDUR RAILWAY, TAL: CHANDUR 6 CHANDUR RAILWAY (M CL) CHANDURRAILWAY AMRAVATI AMRAVATI 0 50 50 RLY, DIST: AMRAVATI GOVT ITI, (ADIVASI), CHIKHALDARA, TAL: 7 CHIKHALDARA (M CL) CHIKHALDARA AMRAVATI AMRAVATI 50 200 250 CHIKHALDARA, DIST: AMRAVATI GOVT ITI, (ADIVASI), DHARNI, TAL: DHARNI, 8 DHARNI(CT) DHARNI AMRAVATI AMRAVATI 50 50 100 DIST: AMRAVATI GOVT ITI, MORSHI, TAL: MORSHI, DIST: 9 MORSHI (M CL) MORSHI AMRAVATI AMRAVATI 0 50 50 AMRAVATI GOVT ITI, NANDGAON KHANDESHWAR, TAL: NANDGAON- 10 NANDGAON-KHANDESHWAR AMRAVATI AMRAVATI 0 50 50 NANDGAON KHANDESHWAR, DIST: AMRAVATI KHANDESHWAR GOVT ITI, MOZARI, TAL: TIOSA, DIST: 11 GURUDEONAGAR TEOSA AMRAVATI AMRAVATI 0 50 50 AMRAVATI GOVT ITI, CHIKHALI, TAL: CHIKHALI, DIST: 12 CHIKHLI (M CL) CHIKHLI BULDANA AMRAVATI 0 50 50 BULDHANA GOVT ITI, DEULGAON RAJA, TAL: DEULGAON 13 DEULGAONRAJA(RURAL) DEOLGAONRAJA BULDANA AMRAVATI 0 50 50 RAJA, DIST: BULDHANA GOVT ITI, KHAMGAON, TAL: KHAMGAON, DIST: 14 KHAMGAON (M CL) KHAMGAON BULDANA AMRAVATI 0 200 200 BULDHANA Hostel Capacity S.N. -

District-Amaravati No.Of Inmates Year of Nature of in Case of Sr
District-Amaravati No.of Inmates Year of Nature of In Case of Sr. Name of the Institutions & Establishment of management Contact Details & E-mail ID Present Hostel,no.of No. Address the Institution/ Total Capacity (Govt.run/aided or Strength SC/ST/OBC Hostel Private) Students 1 Sarswati Gosavi Vidhya Mandir, 9922203306, Madhyamic Ashram Shala, [email protected] 1993 120 120 120 AIDED Nandgaon Peth Tq. & Dist. Amravati 2 Sarswati Gosavi kala va Vidhnyan 9922203306, uchcha Madhyamic Ashram Shala, [email protected] 2009 160 156 156 AIDED Nandgaon Peth Tq. & Dist. Amravati 3 Chaturji Uke Anidanit Adivasi 9561010872, Ashram Shala , Revasa Tq & Dist. [email protected] 2003 280 280 280 AIDED Amravati 4 Anudanit Ashramshala Bhatkuli 9420963527, 1999 400 350 350 Aidedor private hmanuadishabhatkuli@ 5 Jamia Faize Abrar edu and Wel 8956996999, jamiafaizabrar88 2009 137 130 21 private society 6 niwasi matimand vidyalaya, 9545463308, Walgaon Ashti [email protected] 2004 50 35 35 private 7 mahatma phule balgruh Marki 8600922147, [email protected] 1994 20 20 20 govt. run/ granted 8 Sadashanti balgruh, Rundavan 9850445130, colony, Camp Amravati. [email protected] 1992 20 20 20 Adid 9 Holy Cross Home For Babies, 0721-2663861, 1978 60 24 0 Adid Dayasagar, Amravati. [email protected] 10 Ashrit andh karmshala, Jail Cortor 0721-2663438, 1966 50 50 50 Adid Road, Amravati. blindschood.co.in 11 Dr.Narendra Bhivapurkar Andh 0721-2663437, Vidhylaya, Jail cortor Road, blindschool.co.in 1966 115 115 84 Adid Amravati. 12 Pricharya Prashikshan 0721-2553338, Mahavidhylaya Samany Rugnala, [email protected] 1957 48 48 48 Govt. Amravati.