Viola; Violaceae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

"National List of Vascular Plant Species That Occur in Wetlands: 1996 National Summary."
Intro 1996 National List of Vascular Plant Species That Occur in Wetlands The Fish and Wildlife Service has prepared a National List of Vascular Plant Species That Occur in Wetlands: 1996 National Summary (1996 National List). The 1996 National List is a draft revision of the National List of Plant Species That Occur in Wetlands: 1988 National Summary (Reed 1988) (1988 National List). The 1996 National List is provided to encourage additional public review and comments on the draft regional wetland indicator assignments. The 1996 National List reflects a significant amount of new information that has become available since 1988 on the wetland affinity of vascular plants. This new information has resulted from the extensive use of the 1988 National List in the field by individuals involved in wetland and other resource inventories, wetland identification and delineation, and wetland research. Interim Regional Interagency Review Panel (Regional Panel) changes in indicator status as well as additions and deletions to the 1988 National List were documented in Regional supplements. The National List was originally developed as an appendix to the Classification of Wetlands and Deepwater Habitats of the United States (Cowardin et al.1979) to aid in the consistent application of this classification system for wetlands in the field.. The 1996 National List also was developed to aid in determining the presence of hydrophytic vegetation in the Clean Water Act Section 404 wetland regulatory program and in the implementation of the swampbuster provisions of the Food Security Act. While not required by law or regulation, the Fish and Wildlife Service is making the 1996 National List available for review and comment. -

Studies on Ear Fungus-Auricularia from the Woodland of Nameri National Park, Sonitpur District, Assam
International Journal of Interdisciplinary and Multidisciplinary Studies (IJIMS), 2014, Vol 1, No.5, 262-265. 262 Available online at http://www.ijims.com ISSN: 2348 – 0343 Studies on Ear Fungus-Auricularia from the Woodland of Nameri National Park, Sonitpur District, Assam. M.P. Choudhury1*, Dr.T.C Sarma2 1.Department of Botany, Nowgong College, Nagaon -782001, Assam, India. 2.Department of Botany, Gauhati University,Guwahati-7810 14, Assam, India. *Corresponding author: M.P. Choudhury Abstract Auricularia is the genus of the order Auriculariales with more than 10 species. It is also called ear fungus due to its morphological similarities with human ear and has considerable mythological importance. Auricularia auricula is the type species of the order Auriculariales. Different species of Auricularia are edible and some have medicinal importance and still investigations are going on other species to find out their medicinal properties. Extensive woodland of Nameri National Park provides ideal condition for the growth of different species of Auricularia. In this context the present study has been undertaken to study the taxonomy and diversity of different species of Auricularia and bring together information of its ethenomycological uses. As a result of field and laboratory study four different species of Auricularia were collected of which 3 species were identified and one species remain unidentified. Key Words: Auricularia, Taxonomy, Diversity, Nameri National Park. Introduction Auricularia belongs to the order Auriculariales is the largest genus of jelly fungi. They are among the most common and widely distributed members of macrofungi, which generally occurs as saprophytes on wood, logs, branch and twigs causing severe degrees of white rotting of forest trees. -

Eagle Rock Self-Guided Nature Trail
Eagle Rock Self-Guided sages. This evergreen plant has narrow dark green #6 - Dairy Creek, Camp San Luis and West Cuesta the spiny rolled edges. Like other oaks, the Coast leaves and orange-yellow flowers in spring and Ridge Vistas - Take a short break here and enjoy the Live Oak was a major food source for the Chumash Nature Trail summer. The young stems and leaves have been view to West Cuesta Ridge. Burned extensively in Indians. In early spring a golden glow may appear on used as salad greens by Indians. The crushed raw 1994 by the huge 41 these trees. If you take a branch covered with tiny Welcome to the Eagle Rock Self Guided Nature leaves and stems have been used to heal burns and Fire, the Los Padres catkins, you can see the yellow pollen fly on the Trail. This is a 1.8 mile trip to explore the flora, wounds. National Forest is breeze. This is the first step towards the tree making fauna and history of the Chorro Valley. This trail Coyote Bush (Baccharis making a strong acorns. offers great views of Chorro Valley and surrounding pilularis) is a common recovery. Many new hillsides. Take your time and enjoy your outdoor shrub of chaparral. It has seedlings have started to #9 - Dusky-footed Woodrat (Neotoma fuscipes) adventure. small leathery leaves 1/2 grow, including the Nests - As you look around you will see what seem If at the end of your adventure and you no longer to 1 inch long, with a Sergeant Cypress to be piles of debris up to 6 feet high. -

Plant Expedition to the Republic of Georgia
PLANT EXPEDITION TO THE REPUBLIC OF GEORGIA — CAUCASUS MOUNTAINS AUGUST 15 - SEPTEMBER 11, 2010 SPONSORED BY THE DANIEL F. AND ADA L. RICE FOUNDATION PLANT COLLECTING COLLABORATIVE (PCC) Chicago Botanic Garden Missouri Botanical Garden The Morton Arboretum New York Botanical Garden University of Minnesota Landscape Arboretum 1 Table of Contents Summary 3 Georgia’s Caucasus 4-6 Expedition, Expedition Route & Itinerary 7-10 Collaboration 11 Observations 12-13 Documentation 14 Institutional review 14-15 Acknowledgements 16 Maps of the Republic of Georgia and PCC member locations 17 Photo Gallery Collecting 18-19 Collections 20-24 Seed Processing 25 Landscapes 26-29 Transportation 30 Dining 31 People 32-33 Georgia Past and Present 34 Georgia News 35-36 Appendix I – Germplasm Collections Listed by Habit Appendix II – Germplasm Collections Listed Alphabetically Appendix III – Weed Risk Assessment Appendix IV – Field Notes 2 Summary With generous support from the Daniel F. and Ada L. Rice Foundation, Galen Gates and the Plant Collecting Collaborative (PCC) team made outstanding progress through an expedition in the Republic of Georgia. On this recent trip into the Caucasus Moun- tains, a record was set for the most collections made on any Chicago Botanic Garden and PCC expedition to date. The trip, door to door, was 26 days with field collecting most days; nearly every night‘s activity included seed cleaning. We made three hundred collections at 60 sites. Most were seeds from 246 types of trees, shrubs, and perennials, 14 were bulb taxa and four were in the form of perennial roots. Remarkably, 53 taxa are new to U.S. -

History and Cultivation of Parma Violets (Viola, Violaceae) in the United Kingdom and France in the Nineteenth Century
History AND Cultivation OF PARMA VIOLETS (VIOLA, VIOLACEAE) IN THE UNITED KINGDOM AND FRANCE IN THE NINETEENTH Century LUÍS MENDONÇA DE CARVALHO,1,2 FRANCISCA MARIA FERNANDES,3 MARIA DE FÁTIMA NUNES,3 JOÃO BRIGOLA,3 NATHALIE CASBAS,4 AND CLIVE GROVES5 Abstract. Scented cultivars of Parma violets were among the most important urban plants during the nineteenth century, with many references in literature, fashion, art and flower trade. Our research analyzed data related to Parma violets in France and in the United Kingdom and presents the cultivars introduced during the nineteenth century. Keywords: Parma violets, Viola cultivars, United Kingdom, France, social history The first records describing the use of violets origin. Earlier Italian literature from the XVI (Viola L., Violaceae) in Europe are from Ancient century refers to violets with a strong fragrance Greece: fragrant violets were sold in the and pleiomerous flowers that were obtained Athenian agora; praised by Greek poets, as in the from the East, near the city of Constantinople writings of Sappho (Jesus, 2009); used in (now Istanbul), capital of the former Byzantine medicine (Theophrastus, 1916); had an active role Empire. These texts describe violets that looked in myths, such as in the abduction of Persephone like roses, probably referring to ancestral plants (Ovid, 1955; Grimal, 1996); were used in gar- of the contemporary Parma violets (Malécot et lands (Goody, 1993) and present in the Odyssey’s al., 2007). In Naples (Italy), a local tradition garden of Calypso (Homer, 2003). They proposes that these violets came from Portugal, continued to be used throughout the Middle Ages brought by the Bourbon royal family during the and were present in Renaissance herbals (Cleene XVIII century; hence the local name Violetta and Lejeune, 2002). -

Native Trees of Georgia
1 NATIVE TREES OF GEORGIA By G. Norman Bishop Professor of Forestry George Foster Peabody School of Forestry University of Georgia Currently Named Daniel B. Warnell School of Forest Resources University of Georgia GEORGIA FORESTRY COMMISSION Eleventh Printing - 2001 Revised Edition 2 FOREWARD This manual has been prepared in an effort to give to those interested in the trees of Georgia a means by which they may gain a more intimate knowledge of the tree species. Of about 250 species native to the state, only 92 are described here. These were chosen for their commercial importance, distribution over the state or because of some unusual characteristic. Since the manual is intended primarily for the use of the layman, technical terms have been omitted wherever possible; however, the scientific names of the trees and the families to which they belong, have been included. It might be explained that the species are grouped by families, the name of each occurring at the top of the page over the name of the first member of that family. Also, there is included in the text, a subdivision entitled KEY CHARACTERISTICS, the purpose of which is to give the reader, all in one group, the most outstanding features whereby he may more easily recognize the tree. ACKNOWLEDGEMENTS The author wishes to express his appreciation to the Houghton Mifflin Company, publishers of Sargent’s Manual of the Trees of North America, for permission to use the cuts of all trees appearing in this manual; to B. R. Stogsdill for assistance in arranging the material; to W. -

Nutrition Facts N *Learn About Fiber on Page 2
Health and Learning Success Go Hand-In-Hand California’s geography offers a bounty of fresh produce and recreational areas. From stone fruits and salad greens to state and local parks, there is no shortage of healthy foods to eat and outdoor activities to do in California. Studies show that healthy eating and physical activity are correlated with improved academic achievement. Use Harvest of the Month to allow students to experience California-grown fruit and vegetables with their senses. Teach students to live a healthy, active lifestyle and support academic content standards to link the classroom, cafeteria, home, and community. Exploring California Peaches: Taste Testing Network for a Healthy California What You Will Need (per group of 8 students): n Four ripe peaches and four ripe nectarines (two each of yellow and white varieties)* n Paring knife and cutting board n Paper towels *Choose peaches and nectarines that are fragrant and firm to slightly soft when pressed. Optional: Sample other stone fruits (cherries, plums, apricots, etc.) with peaches. Activity: n Distribute yellow peaches and nectarines to each student group. n Observe the look, feel, and smell of each; record observations. n Cut open the second yellow fruit; observe the taste and sound and record observations. n Repeat with white peaches and nectarines. n Discuss similarities and differences among the four varieties. n Record students’ favorite variety; share results with school nutrition staff. For more ideas, reference: Nutrition to Grow On, CDE, 2001. Cooking in Class: Reasons to Eat Peaches Peach Smoothies A ½ cup of sliced peaches (about half of Makes 35 tastes at ¼ cup each a medium peach) provides: Ingredients: n A source of vitamin A and vitamin C. -

Orderform.Pdf
2021 SUMMER & FALL TO USE THIS ORDER FORM Send this page with all orders. Fill in all required information. Fill in customer info at top of every page. If faxing - total pages sent Fax to: 1-800-863-7814. CONTENTS CUSTOMER INFORMATION Annuals 1 Herbs 9 Foliage 9 Customer Ferns 10 Succulents 10 Address Perennials 11 City State/Province Zip/Postal Code Effective for shipments between July 1, 2021 Contact & October 31, 2021. Phone # METHOD OF PAYMENT For details, see Terms & Conditions of Sale. 1. Prepayment 2. Charge on Jolly Farmer account (if previously approved) 3. ACH (replaces draft check) 4. Visa MasterCard Give us a call with American Express your card number. Discover (US only) 5. E-Transfer - CANADA ONLY (Send to: [email protected]) CALENDULa – FLOWERING KALE Customer Name Zip/Postal ☑ Mark desired size Fill in desired shipdates Trays Strips ▼ Tag Code Specie Variety 512 288 144 26 51 Qty Annuals 1165 Calendula , Costa Mix 512 288 144 n/a n/a 1800 Dianthus , Coronet Mix 512 288 144 n/a n/a 1805 Dianthus , Coronet Strawberry 512 288 144 n/a n/a 1815 Dianthus , Diana Mix Formula 512 288 144 n/a n/a 1820 Dianthus , Diana Mix Lavendina 512 288 144 n/a n/a 1825 Dianthus , Diana Mix Picotee 512 288 144 n/a n/a 1830 Dianthus , Ideal Select Mix 512 288 144 n/a n/a 1840 Dianthus , Ideal Select Red 512 288 144 n/a n/a 1845 Dianthus , Ideal Select Rose 512 288 144 n/a n/a 1850 Dianthus , Ideal Select Violet 512 288 144 n/a n/a 1852 Dianthus , Ideal Select White 512 288 144 n/a n/a 1855 Dianthus , Ideal Select Whitefire 512 288 -

COST EFFECTIVE PRODUCTION of SPECIALTY CUT FLOWERS By
COST EFFECTIVE PRODUCTION OF SPECIALTY CUT FLOWERS By TODD JASON CAVINS Bachelor of Science Southwestern Oklahoma State University Weatherford, Oklahoma 1997 Submitted to the Faculty of the Graduate College of the Oklahoma State University in partial fulfillment of the requirements for the Degree of MASTER OF SCIENCE December, 1999 COST EFFECTIVE PRODUCTION OF SPECIALTY CUT FLOWERS Thesis Approved: ' 1 Thesis Advisor .. ;.; ,, ( Dean of the Graduate College 11 ACKNOWLEDGEMENTS The purpose of this study was to improve production methods of various specialty cut flower species. Improving production methods allows growers to reduce cost, improve plant quality and earn higher profits. This study involved three research areas of specialty cut flowers. Partial funding was provided by a S.A.R.E. grant and Bear Creek Farm, Stillwater, OK. I would like to thank my principle advisor Dr. John Dole for his encouragement, support, honesty and perseverance. I would like to thank Dr. Janet Cole and Dr. Jim Ownby for serving on my thesis committee. Dr. Cole offered valuable insight and direction towards the research. Dr. Ownby contributed with his wealth of knowledge in plant physiology. A special thanks goes to Vicki Stamback and the gang at Bear Creek Farm. Vicki's experience as a specialty cut flower grower allowed me to gain personal knowledge of the cut flower industry that would not have taken place without her. Vicki's efforts and cooperation greatly improved this study. I want to thank Randall Smith and Leah Aufill for their assistance and plant care. Tim Hooper also contributed by offering his experiences from the floriculture industry and providing stress relieving lunch breaks. -

Habitat Indicator Species
1 Handout 6 – Habitat Indicator Species Habitat Indicator Species The species lists below are laid out by habitats and help you to find out which habitats you are surveying – you will see that some species occur in several different habitats. Key: * Plants that are especially good indicators of that specific habitat Plants found in Norfolk’s woodland Common Name Scientific Name Alder Buckthorn Frangula alnus Aspen Populus tremula Barren Strawberry Potentilla sterilis Bird Cherry Prunus padus Black Bryony Tamus communis Bush Vetch Vicia sepium Climbing Corydalis Ceratocapnos claviculata Common Cow-wheat Melampyrum pratense Early dog violet Viola reichenbachiana Early Purple Orchid Orchis mascula * English bluebell Hyacinthoides non-scripta* * Field Maple Acer campestre* Giant Fescue Festuca gigantea * Goldilocks buttercup Ranunculus auricomus* Great Wood-rush Luzula sylvatica Greater Burnet-saxifrage Pimpinella major Greater Butterfly-orchid Platanthera chlorantha Guelder Rose Viburnum opulus Hairy Wood-rush Luzula pilosa Hairy-brome Bromopsis ramosa Hard Fern Blechnum spicant Hard Shield-fern Polystichum aculeatum * Hart's-tongue Phyllitis scolopendrium* Holly Ilex aquifolium * Hornbeam Carpinus betulus* * Midland Hawthorn Crataegus laevigata* Moschatel Adoxa moschatellina Narrow Buckler-fern Dryopteris carthusiana Opposite-leaved Golden-saxifrage Chrysosplenium oppositifolium * Pendulous Sedge Carex Pendula* Pignut Conopodium majus Polypody (all species) Polypodium vulgare (sensulato) * Primrose Primula vulgaris* 2 Handout 6 – Habitat -
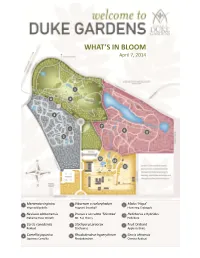
What's in Bloom
WHAT’S IN BLOOM April 7, 2014 5 4 6 2 7 1 9 8 3 12 10 11 1 Mertensia virginica 5 Viburnum x carlcephalum 9 Malus ‘Hopa’ Virginia Bluebells Fragrant Snowball Flowering Crabapple 2 Neviusia alabamensis 6 Prunus x serrulata ‘Shirotae’ 10 Helleborus x hybridus Alabama Snow Wreath Mt. Fuji Cherry Hellebore 3 Cercis canadensis 7 Stachyurus praecox 11 Fruit Orchard Redbud Stachyurus Apple cultivars 4 Camellia japonica 8 Rhododendron hyperythrum 12 Cercis chinensis Japanese Camellia Rhododendron Chinese Redbud WHAT’S IN BLOOM April 7, 2014 BLOMQUIST GARDEN OF NATIVE PLANTS Amelanchier arborea Common Serviceberry Sanguinaria canadensis Bloodroot Cornus florida Flowering Dogwood Stylophorum diphyllum Celandine Poppy Thalictrum thalictroides Rue Anemone Fothergilla major Fothergilla Trillium decipiens Chattahoochee River Trillium Hepatica nobilis Hepatica Trillium grandiflorum White Trillium Hexastylis virginica Wild Ginger Hexastylis minor Wild Ginger Trillium pusillum Dwarf Wakerobin Illicium floridanum Florida Anise Tree Trillium stamineum Blue Ridge Wakerobin Malus coronaria Sweet Crabapple Uvularia sessilifolia Sessileleaf Bellwort Mertensia virginica Virginia Bluebells Pachysandra procumbens Allegheny spurge Prunus americana American Plum DORIS DUKE CENTER GARDENS Camellia japonica Japanese Camellia Pulmonaria ‘Diana Clare’ Lungwort Cercis canadensis Redbud Prunus persica Flowering Peach Puschkinia scilloides Striped Squill Cercis chinensis Redbud Sanguinaria canadensis Bloodroot Clematis armandii Evergreen Clematis Spiraea prunifolia Bridalwreath -

The Evolution of Pollinator–Plant Interaction Types in the Araceae
BRIEF COMMUNICATION doi:10.1111/evo.12318 THE EVOLUTION OF POLLINATOR–PLANT INTERACTION TYPES IN THE ARACEAE Marion Chartier,1,2 Marc Gibernau,3 and Susanne S. Renner4 1Department of Structural and Functional Botany, University of Vienna, 1030 Vienna, Austria 2E-mail: [email protected] 3Centre National de Recherche Scientifique, Ecologie des Foretsˆ de Guyane, 97379 Kourou, France 4Department of Biology, University of Munich, 80638 Munich, Germany Received August 6, 2013 Accepted November 17, 2013 Most plant–pollinator interactions are mutualistic, involving rewards provided by flowers or inflorescences to pollinators. An- tagonistic plant–pollinator interactions, in which flowers offer no rewards, are rare and concentrated in a few families including Araceae. In the latter, they involve trapping of pollinators, which are released loaded with pollen but unrewarded. To understand the evolution of such systems, we compiled data on the pollinators and types of interactions, and coded 21 characters, including interaction type, pollinator order, and 19 floral traits. A phylogenetic framework comes from a matrix of plastid and new nuclear DNA sequences for 135 species from 119 genera (5342 nucleotides). The ancestral pollination interaction in Araceae was recon- structed as probably rewarding albeit with low confidence because information is available for only 56 of the 120–130 genera. Bayesian stochastic trait mapping showed that spadix zonation, presence of an appendix, and flower sexuality were correlated with pollination interaction type. In the Araceae, having unisexual flowers appears to have provided the morphological precon- dition for the evolution of traps. Compared with the frequency of shifts between deceptive and rewarding pollination systems in orchids, our results indicate less lability in the Araceae, probably because of morphologically and sexually more specialized inflorescences.