Parkinson's Disease in Romania
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
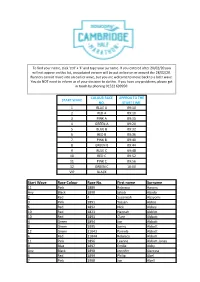
Start Wave Race Colour Race No. First Name Surname
To find your name, click 'ctrl' + 'F' and type your surname. If you entered after 20/02/20 you will not appear on this list, an updated version will be put online on or around the 28/02/20. Runners cannot move into an earlier wave, but you are welcome to move back to a later wave. You do NOT need to inform us of your decision to do this. If you have any problems, please get in touch by phoning 01522 699950. COLOUR RACE APPROX TO THE START WAVE NO. START TIME 1 BLUE A 09:10 2 RED A 09:10 3 PINK A 09:15 4 GREEN A 09:20 5 BLUE B 09:32 6 RED B 09:36 7 PINK B 09:40 8 GREEN B 09:44 9 BLUE C 09:48 10 RED C 09:52 11 PINK C 09:56 12 GREEN C 10:00 VIP BLACK Start Wave Race Colour Race No. First name Surname 11 Pink 1889 Rebecca Aarons Any Black 1890 Jakob Abada 2 Red 4 Susannah Abayomi 3 Pink 1891 Yassen Abbas 6 Red 1892 Nick Abbey 10 Red 1823 Hannah Abblitt 10 Red 1893 Clare Abbott 4 Green 1894 Jon Abbott 8 Green 1895 Jonny Abbott 12 Green 11043 Pamela Abbott 6 Red 11044 Rebecca Abbott 11 Pink 1896 Leanne Abbott-Jones 9 Blue 1897 Emilie Abby Any Black 1898 Jennifer Abecina 6 Red 1899 Philip Abel 7 Pink 1900 Jon Abell 10 Red 600 Kirsty Aberdein 6 Red 11045 Andrew Abery Any Black 1901 Erwann ABIVEN 11 Pink 1902 marie joan ablat 8 Green 1903 Teresa Ablewhite 9 Blue 1904 Ahid Abood 6 Red 1905 Alvin Abraham 9 Blue 1906 Deborah Abraham 6 Red 1907 Sophie Abraham 1 Blue 11046 Mitchell Abrams 4 Green 1908 David Abreu 11 Pink 11047 Kathleen Abuda 10 Red 11048 Annalisa Accascina 4 Green 1909 Luis Acedo 10 Red 11049 Vikas Acharya 11 Pink 11050 Catriona Ackermann -

Surnames 198
Surnames 198 P PACQUIN PAGONE PALCISCO PACUCH PAHACH PALEK PAAHANA PACY PAHEL PALENIK PAAR PADASAK PAHUSZKI PALERMO PAASSARELLI PADDOCK PAHUTSKY PALESCH PABALAN PADELL PAINE PALGUTA PABLIK PADGETT PAINTER PALI PABRAZINSKY PADLO PAIRSON PALILLA PABST PADUNCIC PAISELL PALINA PACCONI PAESANI PAJAK PALINO PACE PAESANO PAJEWSKI PALINSKI PACEK PAFFRATH PAKALA PALKO PACELLI PAGANI PAKOS PALL PACEY PAGANO PALACE PALLO PACHARKA PAGDEN PALADINO PALLONE PACIFIC PAGE PALAGGO PALLOSKY PACILLA PAGLARINI PALAIC PALLOTTINI PACINI PAGLIARINI PALANIK PALLOZZI PACK PAGLIARNI PALANKEY PALM PACKARD PAGLIARO PALANKI PALMA PACKER PAGLIARULO PALAZZONE PALMER PACNUCCI PAGLIASOTTI PALCHESKO PALMERO PACOLT PAGO PALCIC PALMERRI Historical & Genealogical Society of Indiana County 1/21/2013 Surnames 199 PALMIERI PANCIERRA PAOLO PARDUS PALMISANO PANCOAST PAONE PARE PALMISCIANO PANCZAK PAPAKIE PARENTE PALMISCNO PANDAL PAPCIAK PARENTI PALMO PANDULLO PAPE PARETTI PALOMBO PANE PAPIK PARETTO PALONE PANGALLO PAPOVICH PARFITT PALSGROVE PANGBURN PAPPAL PARHAM PALUCH PANGONIS PAPSON PARILLO PALUCHAK PANIALE PAPUGA PARIS PALUDA PANKOVICH PAPURELLO PARISE PALUGA PANKRATZ PARADA PARISEY PALUGNACK PANNACHIA PARANA PARISH PALUMBO PANNEBAKER PARANIC PARISI PALUS PANONE PARAPOT PARISO PALUSKA PANOSKY PARATTO PARIZACK PALYA PANTALL PARCELL PARK PAMPE PANTALONE PARCHINSKY PARKE PANAIA PANTANI PARCHUKE PARKER PANASCI PANTANO PARDEE PARKES PANASKI PANTZER PARDINI PARKHILL PANCHICK PANZY PARDO PARKHURST PANCHIK PAOLINELLIE PARDOE PARKIN Historical & Genealogical Society of Indiana County -

(12) United States Patent (10) Patent No.: US 8.598,119 B2 Mates Et Al
US008598119B2 (12) United States Patent (10) Patent No.: US 8.598,119 B2 Mates et al. (45) Date of Patent: Dec. 3, 2013 (54) METHODS AND COMPOSITIONS FOR AOIN 43/00 (2006.01) SLEEP DSORDERS AND OTHER AOIN 43/46 (2006.01) DSORDERS AOIN 43/62 (2006.01) AOIN 43/58 (2006.01) (75) Inventors: Sharon Mates, New York, NY (US); AOIN 43/60 (2006.01) Allen Fienberg, New York, NY (US); (52) U.S. Cl. Lawrence Wennogle, New York, NY USPC .......... 514/114: 514/171; 514/217: 514/220; (US) 514/229.5: 514/250 (58) Field of Classification Search (73) Assignee: Intra-Cellular Therapies, Inc. NY (US) None See application file for complete search history. (*) Notice: Subject to any disclaimer, the term of this patent is extended or adjusted under 35 (56) References Cited U.S.C. 154(b) by 215 days. U.S. PATENT DOCUMENTS (21) Appl. No.: 12/994,560 6,552,017 B1 4/2003 Robichaud et al. 2007/0203120 A1 8, 2007 McDevitt et al. (22) PCT Filed: May 27, 2009 FOREIGN PATENT DOCUMENTS (86). PCT No.: PCT/US2O09/OO3261 S371 (c)(1), WO WOOOf77OO2 * 6, 2000 (2), (4) Date: Nov. 24, 2010 OTHER PUBLICATIONS (87) PCT Pub. No.: WO2009/145900 Rye (Sleep Disorders and Parkinson's Disease, 2000, accessed online http://www.waparkinsons.org/edu research/articles/Sleep PCT Pub. Date: Dec. 3, 2009 Disorders.html), 2 pages.* Alvir et al. Clozapine-Induced Agranulocytosis. The New England (65) Prior Publication Data Journal of Medicine, 1993, vol. 329, No. 3, pp. 162-167.* US 2011/0071080 A1 Mar. -

Diagnosis and Treatment of Parkinson Disease: Molecules to Medicine
Diagnosis and treatment of Parkinson disease: molecules to medicine Joseph M. Savitt, … , Valina L. Dawson, Ted M. Dawson J Clin Invest. 2006;116(7):1744-1754. https://doi.org/10.1172/JCI29178. Science in Medicine Parkinson disease (PD) is a relatively common disorder of the nervous system that afflicts patients later in life with tremor, slowness of movement, gait instability, and rigidity. Treatment of these cardinal features of the disease is a success story of modern science and medicine, as a great deal of disability can be alleviated through the pharmacological correction of brain dopamine deficiency. Unfortunately these therapies only provide temporary, though significant, relief from early symptoms and do not halt disease progression. In addition, pathological changes outside of the motor system leading to cognitive, autonomic, and psychiatric symptoms are not sufficiently treated by current therapies. Much as the discovery of dopamine deficiency led to powerful treatments for motor symptoms, recent discoveries concerning the role of specific genes in PD pathology will lead to the next revolution in disease therapy. Understanding why and how susceptible cells in motor and nonmotor regions of the brain die in PD is the first step toward preventing this cell death and curing or slowing the disease. In this review we discuss recent discoveries in the fields of diagnosis and treatment of PD and focus on how a better understanding of disease mechanisms gained through the study of monogenetic forms of PD has provided novel therapeutic targets. Find the latest version: https://jci.me/29178/pdf Science in medicine Diagnosis and treatment of Parkinson disease: molecules to medicine Joseph M. -

Advances in Non-Dopaminergic Treatments for Parkinson's Disease
REVIEW ARTICLE published: 22 May 2014 doi: 10.3389/fnins.2014.00113 Advances in non-dopaminergic treatments for Parkinson’s disease Sandy Stayte 1,2 and Bryce Vissel 1,2* 1 Neuroscience Department, Neurodegenerative Disorders Laboratory, Garvan Institute of Medical Research, Sydney, NSW, Australia 2 Faculty of Medicine, University of New South Wales, Sydney, NSW, Australia Edited by: Since the 1960’s treatments for Parkinson’s disease (PD) have traditionally been directed Eero Vasar, University of Tartu, to restore or replace dopamine, with L-Dopa being the gold standard. However, chronic Estonia L-Dopa use is associated with debilitating dyskinesias, limiting its effectiveness. This has Reviewed by: resulted in extensive efforts to develop new therapies that work in ways other than Andrew Harkin, Trinity College Dublin, Ireland restoring or replacing dopamine. Here we describe newly emerging non-dopaminergic Sulev Kõks, University of Tartu, therapeutic strategies for PD, including drugs targeting adenosine, glutamate, adrenergic, Estonia and serotonin receptors, as well as GLP-1 agonists, calcium channel blockers, iron Pille Taba, Universoty of Tartu, chelators, anti-inflammatories, neurotrophic factors, and gene therapies. We provide a Estonia Pekka T. Männistö, University of detailed account of their success in animal models and their translation to human clinical Helsinki, Finland trials. We then consider how advances in understanding the mechanisms of PD, genetics, *Correspondence: the possibility that PD may consist of multiple disease states, understanding of the Bryce Vissel, Neuroscience etiology of PD in non-dopaminergic regions as well as advances in clinical trial design Department, Neurodegenerative will be essential for ongoing advances. We conclude that despite the challenges ahead, Disorders Laboratory, Garvan Institute of Medical Research, patients have much cause for optimism that novel therapeutics that offer better disease 384 Victoria Street, Darlinghurst, management and/or which slow disease progression are inevitable. -

British Family Names
cs 25o/ £22, Cornrll IBniwwitg |fta*g BOUGHT WITH THE INCOME FROM THE SAGE ENDOWMENT FUND THE GIFT OF Hcnrti W~ Sage 1891 A.+.xas.Q7- B^llll^_ DATE DUE ,•-? AUG 1 5 1944 !Hak 1 3 1^46 Dec? '47T Jan 5' 48 ft e Univeral, CS2501 .B23 " v Llb«"y Brit mii!Sm?nS,£& ori8'" and m 3 1924 olin 029 805 771 The original of this book is in the Cornell University Library. There are no known copyright restrictions in the United States on the use of the text. http://www.archive.org/details/cu31924029805771 BRITISH FAMILY NAMES. : BRITISH FAMILY NAMES ftbetr ©riain ano fIDeaning, Lists of Scandinavian, Frisian, Anglo-Saxon, and Norman Names. HENRY BARBER, M.D. (Clerk), "*• AUTHOR OF : ' FURNESS AND CARTMEL NOTES,' THE CISTERCIAN ABBEY OF MAULBRONN,' ( SOME QUEER NAMES,' ' THE SHRINE OF ST. BONIFACE AT FULDA,' 'POPULAR AMUSEMENTS IN GERMANY,' ETC. ' "What's in a name ? —Romeo and yuliet. ' I believe now, there is some secret power and virtue in a name.' Burton's Anatomy ofMelancholy. LONDON ELLIOT STOCK, 62, PATERNOSTER ROW, E.C. 1894. 4136 CONTENTS. Preface - vii Books Consulted - ix Introduction i British Surnames - 3 nicknames 7 clan or tribal names 8 place-names - ii official names 12 trade names 12 christian names 1 foreign names 1 foundling names 1 Lists of Ancient Patronymics : old norse personal names 1 frisian personal and family names 3 names of persons entered in domesday book as HOLDING LANDS temp. KING ED. CONFR. 37 names of tenants in chief in domesday book 5 names of under-tenants of lands at the time of the domesday survey 56 Norman Names 66 Alphabetical List of British Surnames 78 Appendix 233 PREFACE. -

The Use of Stems in the Selection of International Nonproprietary Names (INN) for Pharmaceutical Substances
WHO/PSM/QSM/2006.3 The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances 2006 Programme on International Nonproprietary Names (INN) Quality Assurance and Safety: Medicines Medicines Policy and Standards The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances FORMER DOCUMENT NUMBER: WHO/PHARM S/NOM 15 © World Health Organization 2006 All rights reserved. Publications of the World Health Organization can be obtained from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: [email protected]). Requests for permission to reproduce or translate WHO publications – whether for sale or for noncommercial distribution – should be addressed to WHO Press, at the above address (fax: +41 22 791 4806; e-mail: [email protected]). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. -

Most-Common-Surnames-Bmd-Registers-16.Pdf
Most Common Surnames Surnames occurring most often in Scotland's registers of Births, Marriages and Deaths Counting only the surname of the child for births, the surnames of BOTH PARTIES (for example both BRIDE and GROOM) for marriages, and the surname of the deceased for deaths Note: the surnames from these registers may not be representative of the surnames of the population of Scotland as a whole, as (a) they include the surnames of non-residents who were born / married / died here; (b) they exclude the surnames of residents who were born / married / died elsewhere; and (c) some age-groups have very low birth, marriage and death rates; others account for most births, marriages and deaths.ths Registration Year = 2016 Position Surname Number 1 SMITH 2056 2 BROWN 1435 3 WILSON 1354 4 CAMPBELL 1147 5 STEWART 1139 6 THOMSON 1127 7 ROBERTSON 1088 8 ANDERSON 1001 9 MACDONALD 808 10 TAYLOR 782 11 SCOTT 771 12 REID 755 13 MURRAY 754 14 CLARK 734 15 WATSON 642 16 ROSS 629 17 YOUNG 608 18 MITCHELL 601 19 WALKER 589 20= MORRISON 587 20= PATERSON 587 22 GRAHAM 569 23 HAMILTON 541 24 FRASER 529 25 MARTIN 528 26 GRAY 523 27 HENDERSON 522 28 KERR 521 29 MCDONALD 520 30 FERGUSON 513 31 MILLER 511 32 CAMERON 510 33= DAVIDSON 506 33= JOHNSTON 506 35 BELL 483 36 KELLY 478 37 DUNCAN 473 38 HUNTER 450 39 SIMPSON 438 40 MACLEOD 435 41 MACKENZIE 434 42 ALLAN 432 43 GRANT 429 44 WALLACE 401 45 BLACK 399 © Crown Copyright 2017 46 RUSSELL 394 47 JONES 392 48 MACKAY 372 49= MARSHALL 370 49= SUTHERLAND 370 51 WRIGHT 357 52 GIBSON 356 53 BURNS 353 54= KENNEDY 347 -

(12) Patent Application Publication (10) Pub. No.: US 2015/0072964 A1 Mates Et Al
US 20150.072964A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2015/0072964 A1 Mates et al. (43) Pub. Date: Mar. 12, 2015 (54) NOVELMETHODS filed on Apr. 14, 2012, provisional application No. 61/624.293, filed on Apr. 14, 2012, provisional appli (71) Applicant: INTRA-CELLULAR THERAPIES, cation No. 61/671,713, filed on Jul. 14, 2012, provi INC., New York, NY (US) sional application No. 61/671,723, filed on Jul. 14, 2012. (72) Inventors: Sharon Mates, New York, NY (US); Robert Davis, New York, NY (US); Publication Classification Kimberly Vanover, New York, NY (US); Lawrence Wennogle, New York, (51) Int. Cl. NY (US) C07D 47L/6 (2006.01) A613 L/4985 (2006.01) (21) Appl. No.: 14/394,469 A6II 45/06 (2006.01) (52) U.S. Cl. (22) PCT Fled: Apr. 14, 2013 CPC .............. C07D 471/16 (2013.01); A61K 45/06 (2013.01); A61 K3I/4985 (2013.01) (86) PCT NO.: PCT/US13A36512 USPC ...... 514/171; 514/250; 514/211.13: 514/217; S371 (c)(1), 514/214.02: 514/220 (2) Date: Oct. 14, 2014 (57) ABSTRACT Use of particular substituted heterocycle fused gamma-car Related U.S. Application Data boline compounds as pharmaceuticals for the treatment of (60) Provisional application No. 61/624.291, filed on Apr. agitation, aggressive behaviors, posttraumatic stress disorder 14, 2012, provisional application No. 61/624,292, or impulse control disorders. US 2015/0072964 A1 Mar. 12, 2015 NOVEL METHODS One type of ICD is Intermittent Explosive Disorder (IED) which involves violence or rage. There is a loss of control CROSS REFERENCE TO RELATED grossly out of proportion to any precipitating psychosocial APPLICATIONS stresses. -

Hebron Maple Festival This Weekend RHAM Juniors Hold Fashion Show
★ ★ ★ ★ ★ US. POSTAGE PAID POSTAL CUSTOMER GLASTONBURY CITIZEN, INC. LOCAL RIVEREAST PRESORTED STANDARD NewsServing Amston, Andover, Cobalt, East Hampton, Hebron,Bulletin Marlborough, Middle Haddam, Portland, Colchester and Salem Volume 33, Number 51 Published by The Glastonbury Citizen March 13, 2009 Hebron Maple Festival This Weekend by Sarah McCoy The steel buckets hanging from the sides of explained that the ideal syrup-making weather trees can only mean one thing. With spring just features nighttime temperatures in the low 20s, around the corner, the time has come for the with daytime highs in the 40s, with lots of sun 19th annual Hebron Maple Festival. and little to no wind. While this year’s season The festival will be held this year Saturday started off terribly, both sugarers agreed that and Sunday, March 14 and 15, from 10 a.m.-4 the past few weeks have made up for the slow p.m. both days. The affair will feature events start. “This year might prove to be average,” across the town and plenty of food. Palmer said. “That would be a huge achieve- “It’s unique,” Wayne Palmer, owner of ment considering how the season started.” Winding Brook Sugar House and one of the Palmer places taps in over 850 trees. Last coordinators for this year’s festival, said. year, he said, he produced 250 gallons of syrup. “We’re the only town in Connecticut that does “Last year was one of the best in history,” a maple festival and I’ve been told it rivals any- Palmer said. “It was an anomaly but we could thing in Vermont because we’ve kept money get to 200 this year.” out of the equation.” In addition to its use on pancakes and As in years past, there is free admission to waffles, syrup can be used for all different rea- the Maple Festival. -

Effects of Serotonin 5-HT1A Agonist in Advanced Parkinson's Disease
Movement Disorders Vol. 20, No. 8, 2005, pp. 932–936 © 2005 Movement Disorder Society Effects of Serotonin 5-HT1A Agonist in Advanced Parkinson’s Disease William Bara-Jimenez, MD,* Francesco Bibbiani, MD, Michael J. Morris, MD, Tzvetelina Dimitrova, MD, Abdullah Sherzai, MD, Maral M. Mouradian, MD, and Thomas N. Chase, MD Experimental Therapeutics Branch, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, Maryland, USA Abstract: Intermittent stimulation of striatal dopaminergic re- double-blind, placebo-controlled, proof-of-concept study. Sar- ceptors seems to contribute to motor dysfunction in advanced izotan alone or with intravenous levodopa had no effect on Parkinson’s disease (PD). With severe dopaminergic denerva- parkinsonian severity. But at safe and tolerable doses, sarizotan tion, exogenous levodopa is largely decarboxylated to dopa- coadministration reduced levodopa-induced dyskinesias and mine in serotonergic terminals. If 5-HT1A autoreceptors regu- prolonged its antiparkinsonian response (P Յ 0.05). Under the late dopamine as well as serotonin release, in parkinsonian conditions of this study, our findings suggest that 5-HT1A patients inhibition of striatal serotonergic neuron firing might receptor stimulation in levodopa-treated parkinsonian patients help maintain more physiological intrasynaptic dopamine con- can modulate striatal dopaminergic function and that 5-HT1A centrations and thus ameliorate motor fluctuations and dyski- agonists may be useful as levodopa adjuvants in the -

Proquest Dissertations
LITERATURE, MODERNITY, NATION THE CASE OF ROMANIA, 1829-1890 Alexander Drace-Francis School of Slavonic and East European Studies, University College London Thesis submitted for the degree of PhD June, 2001 ProQuest Number: U642911 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. uest. ProQuest U642911 Published by ProQuest LLC(2016). Copyright of the Dissertation is held by the Author. All rights reserved. This work is protected against unauthorized copying under Title 17, United States Code. Microform Edition © ProQuest LLC. ProQuest LLC 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 48106-1346 ABSTRACT The subject of this thesis is the development of a literary culture among the Romanians in the period 1829-1890; the effect of this development on the Romanians’ drive towards social modernization and political independence; and the way in which the idea of literature (as both concept and concrete manifestation) and the idea of the Romanian nation shaped each other. I concentrate on developments in the Principalities of Moldavia and Wallachia (which united in 1859, later to form the old Kingdom of Romania). I begin with an outline of general social and political change in the Principalities in the period to 1829, followed by an analysis of the image of the Romanians in European public opinion, with particular reference to the state of cultural institutions (literacy, literary activity, education, publishing, individual groups) and their evaluation for political purposes.