Final Project No.: 73185 VTRC 05
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Summary of Public Comments to State Water Control Board Adequacy of NWP 12 to Ensure Compliance with State Standards
Summary of Public Comments to State Water Control Board Adequacy of NWP 12 to Ensure Compliance with State Standards Prepared by Wild Virginia Dominion Pipeline Monitoring Coalition (DPMC) August 15, 2018 Table of Contents Executive Summary . 7 I. Overall Body of Comments and Organization of the Online Records . 10 II. Waterbodies Discussed . 12 Mountain Valley Pipeline New River Basin Kimballton Branch . 14 (tributary to Stony Creek) Stony Creek . 15 (aka Big Stony Creek - tributary to New River) Little Stony Creek . 15 (tributary to New River) Doe Creek . 16 (tributary to New River) Greenbriar Branch . 17 (tributary to Sinking Creek) Unnamed Tributary to Grass Run . 17 (tributary to Grass Run) Sinking Creek . 18 (tributary to New River) James River Basin Craig Creek . 19 (tributary to James River) Roanoke River Basin Bottom Creek, Mill Creek, and Tributaries . 20 (tributary to South Fork Roanoke River) South Fork Roanoke River . 22 (tributary to Roanoke River) Mill Creek (Montogomery Co.) . 23 (tributary to North Fork Roanoke River) Bottom Spring . 24 (tributary to North Fork Roanoke River) Salmon Spring . 24 (tributary to North Fork Roanoke River) 2 Bradshaw Creek . 25 (tributary to North Fork Roanoke River) Flatwoods Branch . 25 (tributary to North Fork Roanoke River) North Fork Roanoke River . 25 (tributary to Roanoke River) North Fork Blackwater River . 26 (tributary to Blackwater River) Green Creek . 27 (tributary to South Fork Blackwater River) Teels Creek . 27 (tributary to Little Creek) Little Creek . 28 (tributary to Blackwater River) Blackwater River . 28 (tributary to Roanoke River - Smith Mtn. Lake) Pigg River . 29 (tributary to Roanoke River - Leesville Lake) Roanoke River . -

Signal Knob Northern Massanutten Mountain Catback Mountain Browns Run Southern Massanutten Mountain Five Areas of Around 45,000 Acres on the Lee the West
Sherman Bamford To: [email protected] <[email protected] cc: Sherman Bamford <[email protected]> > Subject: NiSource Gas Transmission and Storage draft multi-species habitat conservation plan comments - attachments 2 12/13/2011 03:32 PM Sherman Bamford Forests Committee Chair Virginia Chapter – Sierra Club P.O. Box 3102 Roanoke, Va. 24015 [email protected] (540) 343-6359 December 13, 2011 Regional Director, Midwest Region Attn: Lisa Mandell U.S. Fish and Wildlife Service Ecological Services 5600 American Blvd. West, Suite 990 Bloomington, MN 55437-1458 Email: [email protected] Dear Ms. Mandell: On behalf of the Virginia Chapter of Sierra Club, the following are attachments to our previously submitted comments on the the NiSource Gas Transmission and Storage (“NiSource”) draft multi-species habitat conservation plan (“HCP”) and the U.S. Fish & Wildlife Service (“Service”) draft environmental impact statement (“EIS”). Draft of Virginia Mountain Treasures For descriptions and maps only. The final version was published in 2008. Some content may have changed between 2007 and 2008. Sherman Bamford Sherman Bamford PO Box 3102 Roanoke, Va. 24015-1102 (540) 343-6359 [email protected] Virginia’s Mountain Treasures ART WORK DRAWING The Unprotected Wildlands of the George Washington National Forest A report by the Wilderness Society Cover Art: First Printing: Copyright by The Wilderness Society 1615 M Street, NW Washington, DC 20036 (202)-843-9453 Wilderness Support Center 835 East Second Avenue Durango, CO 81302 (970) 247-8788 Founded in 1935, The Wilderness Society works to protect America’s wilderness and to develop a nation- wide network of wild lands through public education, scientific analysis, and advocacy. -

SR V18 Index.Pdf (969.3Kb)
Index to Volume 18 Rachael Garrity A Biedma, 115 A Girls Life Before the War, 81 Bimini, 119 Adams, President John, I 0, 13-14 Blacksburg, Va., 25, 30, 57, 80, 82-85, 91 Alabama, IOI Blue Ridge Mountains, 82 Alien and Sedition Acts, I 0 Boston, Barbara, 112 Alonso de Chavez, 115 Botetourt County, Va., 25, 27, 29, 54, 63, Alonso de Santa Cruz, 111-113 65, 79 Allegheny Mountains, 82 Bouleware, Jane Grace Preston, 78 American Declaration of Independence, 9 Boulware, Aubin Lee, 78 American Indians, 4, 52-54, 58, 66, 82, Bradenton, Fla., I 06 100, 105, 107-108 Brady, Mathew, 84 American Revolution, 58, 72, 73 Braham, John, 34 Apafalaya, I 0 I Brain, Jeffrey, I 05 Appalachia, I 0 I, I 04 Breckenridge, Robert, 40 Appalachian Mountains, 3, 20, 55 Brissot, Jacques Pierre, 16 Appalachian Trail, 110 Bristol, Tenn.Na., 99, I 09, 124 Arkansas, I 05 Bristol News, 126-127 Augusta County, Va., 25-27, 30, 33, 35, British North America, 55 37-38,40,43-44,52,54, 71 Brown, John, 91, 135 Avenel House Plantation, 81 Brown, John Mason, 135 Brown, John Meredith, 135 B Brunswick County, Va., 40 Bahaman Channel, 118-119 Buchanan, Colonel John, 27, 30, 35, 41, Baird (Beard), John, 40-41 52-53 Baldwin, Caroline (Cary) Marx, 78 Buchanan, Va., 27, 38, 52-53 Bank of the United States, 6 Bullett, Captain, 62-65 Bardstown, Ky., 3, 17 Bullpasture River, 54 Barreis, David, I 05 Bureau ofAmerican Ethnology, 110 Barrens, The, Ky., 13, 17 Burke County, N.C., I 03 Bassett, Colonel, 65 Burwell, Letitia M., 81-83, 86, 90 Batson, Mordecai, 60 Bussell Island, Tenn., 123 Battle -

A Study of Migration from Augusta County, Virginia, to Kentucky, 1777-1800
W&M ScholarWorks Dissertations, Theses, and Masters Projects Theses, Dissertations, & Master Projects 1987 "Peopling the Western Country": A Study of Migration from Augusta County, Virginia, to Kentucky, 1777-1800 Wendy Sacket College of William & Mary - Arts & Sciences Follow this and additional works at: https://scholarworks.wm.edu/etd Part of the United States History Commons Recommended Citation Sacket, Wendy, ""Peopling the Western Country": A Study of Migration from Augusta County, Virginia, to Kentucky, 1777-1800" (1987). Dissertations, Theses, and Masters Projects. Paper 1539625418. https://dx.doi.org/doi:10.21220/s2-ypv2-mw79 This Thesis is brought to you for free and open access by the Theses, Dissertations, & Master Projects at W&M ScholarWorks. It has been accepted for inclusion in Dissertations, Theses, and Masters Projects by an authorized administrator of W&M ScholarWorks. For more information, please contact [email protected]. "PEOPLING THE WESTERN COUNTRY": A STUDY OF MIGRATION FROM AUGUSTA COUNTY, VIRGINIA, TO KENTUCKY, 1777-1800 A Thesis Presented to The Faculty of the Department of History The College of William and Mary in Virginia In Partial Fulfillment Of the Requirements for the Degree of Master of Arts by Wendy Ellen Sacket 1987 APPROVAL SHEET This thesis is submitted in partial fulfillment of the requirements for the degree of Master of Arts Author Approved, December, 1987 John/Se1by *JU Thad Tate ies Whittenburg i i TABLE OF CONTENTS Page ACKNOWLEDGMENTS.............................. iv LIST OF T A B L E S ...............................................v LIST OF MAPS . ............................................. vi ABSTRACT................................................... v i i CHAPTER I. AN INTRODUCTION TO THE LITERATURE, PURPOSE, AND ORGANIZATION OF THE PRESENT STUDY . -

Table of Contents
William2 Gay of the Little Calfpasture 1 PREFACE This is an excerpt from a much longer report which represents my current thinking on the many Scotch- Irish families surnamed Gay who pioneered in the Valley of Virginia (the Shenandoah) in the 1740s. This excerpt is focused on William2 Gay (a.k.a. William-A Gay), one of the two such Williams (the other I call William-B) who settled on the Little Calfpasture River in the present county of Rockbridge. In order to call my subject William2 Gay (implying that his parents were the first immigrants to America of his line), I must logically provide some account of those parents, and this I have done below, although it must be noted that they remain rather shadowy figures—theoretical constructs based largely on onomastic analysis of the child-naming patterns in the families of their putative descendants. The children of this first couple, whom I hypothesize were named John1 and Agnes, were, in probable order of birth: William, James, John, Robert, Samuel, and Eleanor. Of these, besides William, the only one for whom I have included material here from my plenary report is Eleanor, and that only because her inclusion crucially impacts the structure of this family, and also uniquely brings into play evidence of its origins in Ulster—northern Ireland—probably either in the counties of Donegal or Londonderry. The main line of this report, as well as the plenary report from which it is excerpted, consists of a series of linked family sketches, in descendancy order. Each sketch covers one man, or one woman, their spouse or spouses, and their set or sets of children. -
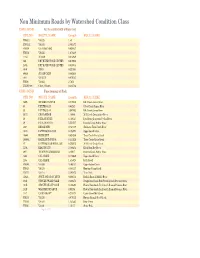
Non Minimum Roads by Watershed Condition Class
Non Minimum Roads by Watershed Condition Class USFS_COND Not Associated with a Watershed RTE_NO ROUTE_NAME Length HUC12_NAME FH561.1 VA.623 1.49 FH561.2 VA.623 2.864371 FH598 VA.818&VA.682 0.020837 FH554 VA.625 1.678439 444C MILAM 0.013939 265 DRY RIVER WORK CENTER 0.019088 265A DRY RIVER WORK CENTER 0.049903 4040 TENN 0.227884 49030 SUGAR CAMP 0.463635 4041 VALLEY 0.076535 FH580 VA.663 2.7653 FH WV589 CO15, CO15/3 0.354756 USFS_COND Functioning at Risk RTE_NO ROUTE_NAME Length HUC12_NAME 765B SNAKE DEN SPUR 0.212038 Elk Creek-James River 35 PETITES GAP 0.03242 Otter Creek-James River 35 PETITES GAP 3.982893 Elk Creek-James River 361C CIGAR RIDGE 1.10046 Mill Creek-Cowpasture River 39 PEDLAR RIVER 6.768137 Lynchburg Reservoir-Pedlar River 39 PEDLAR RIVER 5.554507 Browns Creek-Pedlar River 1037 BROAD RUN 0.747509 Skidmore Fork-North River 104E PATTERSON R-O-W 0.193824 Upper South River 10800 HAZELNUT 0.625286 Trout Creek-Craig Creek 10800A HAZELNUT SPUR A 0.331626 Trout Creek-Craig Creek P7 PATTERSON SPECIAL USE 0.295912 Mill Creek-Craig Creek 225A BLACKS RUN 2.506454 Black Run-Dry River 39E THIRTEEN CROSSINGS 3.42817 Browns Creek-Pedlar River 1283 CAL CLARK 0.172632 Upper South River 1283 CAL CLARK 1.313025 Irish Creek FH589 VA.636 3.465131 Upper Johns Creek FH563 VA.615 8.545137 Hunting Camp Creek FH575 VA.641 2.385472 Tract Fork 1584A SPICK AND SPAN SPUR 0.306516 Buffalo Branch-Middle River 151R STONEY SWAMP SALE 0.322371 Rough Run-South Fork South Branch Potomac River 151R STONEY SWAMP SALE 0.458325 Hawes Run-South Fork South Branch -

Most Effective Basins Funding Allocations Rationale May 18, 2020
Most Effective Basins Funding Allocations Rationale May 18, 2020 U.S. Environmental Protection Agency Chesapeake Bay Program Office Most Effective Basins Funding In the U.S. Environmental Protection Agency’s (EPA) Fiscal Year (FY) 2020 Appropriations Conference Report, an increase to the Chesapeake Bay Program (CBP) Budget was provided in the amount of $6 million for “state-based implementation in the most effective basins.” This document describes the methodology EPA followed to establish the most effective use of these funds and the best locations for these practices to be implemented to make the greatest progress toward achieving water quality standards in the Chesapeake Bay. The most effective basins to reduce the effects of excess nutrient loading to the Bay were determined considering two factors: cost effectiveness and load effectiveness. Cost effectiveness was considered as a factor to assure these additional funds result in state-based implementation of practices that achieve the greatest benefit to water quality overall. It was evaluated by looking at what the jurisdictions have reported in their Phase III Watershed Implementation Plans (WIPs) as the focus of their upcoming efforts, and by looking at the average cost per pound of reduction for BMP implementation by sector. Past analyses of cost per pound of reduction have shown that reducing nitrogen is less costly by far than reducing phosphorus1. Based on that fact, EPA determined that the focus of this evaluation would be to target nitrogen reductions in the watershed. Evaluating the load reduction targets in all the jurisdictions’ Phase III WIPs shows that the agricultural sector is targeted for 86 percent of the overall reductions identified to meet the 2025 targets collectively set by the jurisdictions. -

August 24, 2020 at 5:30 P.M
AT A REGULAR MEETING OF THE ROCKBRIDGE COUNTY BOARD OF SUPERVISORS HELD IN THE ROCKBRIDGE COUNTY ADMINISTRATIVE OFFICE BUILDING AT 150 SOUTH MAIN STREET, LEXINGTON, VIRGINIA ON MONDAY, AUGUST 24, 2020 AT 5:30 P.M. *** Please take notice, that the scheduled 4:30 p.m. Work Session was cancelled. *** BOARD MEMBERS PRESENT: D. E. LYONS R. W. DAY L.E. AYERS D.B. MCDANIEL (Participated Remotely) A.J. “JAY” LEWIS, II. COUNTY ADMINISTRATOR: SPENCER H. SUTER COUNTY ATTORNEY: VICKIE L. HUFFMAN *** CALLED TO ORDER: Chairman Lyons called the meeting to order at 5:30 p.m. Administrative Assistant to the County Administrator Brandy Whitten conducted a roll call of the Board members. All Board members were present, with Supervisor McDaniel participating remotely. Supervisor Lewis offered an invocation for anyone who wished to participate. The Board then led in the Pledge of Allegiance. Chairman Lyons advised of the following announcements: “While the room will be open to the public, due to the size of the meeting room and social distancing requirements, no more than 30 attendees will be allowed into the room at one time. Per the Governor of the Commonwealth of Virginia Executive Order 63, all persons over the age of ten, that do not have a precluding medical condition, are asked to wear a face covering. We will hold citizen comment near the beginning of the meeting. Also, there are several public hearings this evening. We will do our best to take citizen comment remotely. There are two options for citizens to offer comment remotely: 1) To join the Rockbridge County Board of Supervisor’s Zoom webinar The link is available on the County website to copy into the address line on your browser. -

'O'er Mountains and Rivers': Community and Commerce
MCCARTNEY, SARAH ELLEN, Ph.D. ‘O’er Mountains and Rivers’: Community and Commerce in the Greenbrier Valley in the Late Eighteenth Century. (2018) Directed by Dr. Greg O’Brien. 464 pp. In the eighteenth-century Greenbrier River Valley of present-day West Virginia, identity was based on a connection to “place” and the shared experiences of settlement, commerce, and warfare as settlers embraced an identity as Greenbrier residents, Virginians, and Americans. In this dissertation, I consider the Greenbrier Valley as an early American place participating in and experiencing events and practices that took place throughout the American colonies and the Atlantic World, while simultaneously becoming a discrete community and place where these experiences formed a unique Greenbrier identity. My project is the first study of the Greenbrier Valley to situate the region temporally within the revolutionary era and geographically within the Atlantic World. For many decades Greenbrier Valley communities were at the western edge of Virginia’s backcountry settlements in what was often an “ambiguous zone” of European control and settlers moved in and out of the region with the ebb and flow of frontier violence. Settlers arriving in the region came by way of the Shenandoah Valley where they traveled along the Great Wagon Road before crossing into the Greenbrier region through the mountain passes and rivers cutting across the Allegheny Mountains. Without a courthouse or church, which were the typical elements of community in eighteenth- century Virginia society, until after the American Revolution, Greenbrier settlers forged the bonds of their community through other avenues, including the shared hardships of the settlement experience. -

2008 Maury River Biologist Report
Maury River 2008 The Maury River runs 42 miles from the confluence of the Calfpasture River and the Little Calfpasture River near Goshen to its mouth in Glasgow. The entire watershed is in Rockbridge County, Va. The Maury River provides ample angling opportunities, from the trout-stocked rapids of Goshen Pass to the deep pools downstream of Buena Vista. The Department of Game and Inland Fisheries (DGIF) has sampled the Maury River at several established locations since 1997, all downstream of Goshen Pass. The primary objective is to obtain population statistics for sport fish and to manage them appropriately for the anglers of Virginia. Sport fish of the Maury River include stocked brown and rainbow trout that are introduced several times annually by DGIF in the Goshen Pass area. Smallmouth bass, redbreast sunfish, rock bass, and carp are the dominant species throughout the system. Muskellunge and flathead catfish are available in limited number near Glasgow. Below is a location map of four electrofishing stations that have been in use by DGIF for a decade. DGIF Sample Sites Beans Bottom VMI (Rt 60) Sniders Glasgow 2 Smallmouth bass This species is considered the primary target of most anglers that fish the Maury River. Results are published as “catch rates” or number of fish per hour netted by biologists. The line graph on the next page shows that the catch rates for smallmouth bass rivers across Virginia is about the same as the average over time in the Maury River (91 fish per hour). The highest catch rates occur near Glasgow (139 fish/hour) and the lowest near Beans Bottom, above Lexington. -

Thesis Working Plan
Distribution, Genetic Characterization, and Life History of the James spinymussel, Pleurobema collina (Bivalvia: Unionidae), in Virginia and North Carolina by Melissa A. Petty Thesis submitted to the Faculty of the Virginia Polytechnic Institute and State University in partial fulfillment of the requirements for the degree of Master of Science in Fisheries and Wildlife Sciences Approved: ____________________________ _____________________________ Dr. Richard J. Neves, Chairperson Dr. Eric M. Hallerman _________________________ Dr. Paul Angermeier February 11, 2005 Blacksburg, Virginia Keywords: James spinymussel, Pleurobema collina, Distribution, Survey, Life History, DNA Sequences, Fish Host, Phenotype, Morphology, Monophyletic, Genetic Drift Distribution, Genetic Characterization, and Life History of the James spinymussel, Pleurobema collina (Bivalvia: Unionidae), in Virginia and North Carolina by Melissa A. Petty Richard J. Neves, Chairperson Fisheries and Wildlife Sciences (ABSTRACT) Three spined, mussel species occur in the United States along the Atlantic slope; James spinymussel (Pleurobema collina), Tar spinymussel (Elliptio steinstansana), and Altamaha spinymussel (E. spinosa). The James spinymussel was listed as endangered in 1988, and was until recently considered to be endemic to the James River basin (Clarke and Neves 1984; USFWS 1990). Biologists with the North Carolina Department of Transportation (NCDOT) discovered spinymussel populations in the Dan and Mayo rivers in NC in 2000 and 2001, respectively. The U.S. Fish & Wildlife Service (USFWS) tentatively identified this species as Pleurobema collina. My project proposed by the Virginia Cooperative Fish and Wildlife Research Unit to the USFWS and the Virginia Transportation Research Council, determined where P. collina resides in VA and what the extent of its range is within the state. An informal preliminary survey design for P. -

Printable Pdf Version
CHRONOLOGY of CONSTRUCTION, OPERATION, and MAINTENANCE of LAKE MERRIWEATHER and GOSHEN DAM See acronyms, abbreviations and sources at end of chronology record Introduction From the chronology following this introduction, you will see that the problems of water quality, sedimentation, and dam safety are long-standing. Virginia’s SWCB, DCR. DGIF, VDH, and DEQ and the U.S. EPA, USACE, and U.S. FWS have all worked very hard to try and make Goshen Dam safe and the Little Calfpasture River clean. Yet, a permanent, comprehensive and integrated solution still eludes us. For example, in 2013, the primary state dam regulatory agency, Virginia Department of Conservation and Recreation, accepted the recommendation from the National Capital Area Council of the Boy Scouts of America (BSA) that the lake would be lowered for all of the non- camping season. This contradicted the DEQ order to maintain minimum levels to prevent sediment flushing from the lake. Also, in this instance, DCR did not require analysis of alternate management techniques for inspection, removal of debris, tree-planting, and removal of sediment to maintain lake depth that would help protect downstream water quality. In addition, DCR approved the BSA’s Emergency Action Plan without response to public comments. Even so, RACC sent comments to DCR on the Plan in July 2013, mainly expressing the need for a more effective communications plan for informing those on the river when the lake is being significantly lowered and, in the event of a flood and potential overtopping of the dam, a horn alarm. The current system of informing only the Rockbridge county emergency management office – which then issues a text message or email to those who subscribe – has proved to be ineffective and unreliable.