US Naval Warfare Training Range Navy EFH Assessment
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

L the Charlatans UK the Charlatans UK Vs. the Chemical Brothers
These titles will be released on the dates stated below at physical record stores in the US. The RSD website does NOT sell them. Key: E = Exclusive Release L = Limited Run / Regional Focus Release F = RSD First Release THESE RELEASES WILL BE AVAILABLE AUGUST 29TH ARTIST TITLE LABEL FORMAT QTY Sounds Like A Melody (Grant & Kelly E Alphaville Rhino Atlantic 12" Vinyl 3500 Remix by Blank & Jones x Gold & Lloyd) F America Heritage II: Demos Omnivore RecordingsLP 1700 E And Also The Trees And Also The Trees Terror Vision Records2 x LP 2000 E Archers of Loaf "Raleigh Days"/"Street Fighting Man" Merge Records 7" Vinyl 1200 L August Burns Red Bones Fearless 7" Vinyl 1000 F Buju Banton Trust & Steppa Roc Nation 10" Vinyl 2500 E Bastille All This Bad Blood Capitol 2 x LP 1500 E Black Keys Let's Rock (45 RPM Edition) Nonesuch 2 x LP 5000 They's A Person Of The World (featuring L Black Lips Fire Records 7" Vinyl 750 Kesha) F Black Crowes Lions eOne Music 2 x LP 3000 F Tommy Bolin Tommy Bolin Lives! Friday Music EP 1000 F Bone Thugs-N-Harmony Creepin' On Ah Come Up Ruthless RecordsLP 3000 E David Bowie ChangesNowBowie Parlophone LP E David Bowie ChangesNowBowie Parlophone CD E David Bowie I’m Only Dancing (The Soul Tour 74) Parlophone 2 x LP E David Bowie I’m Only Dancing (The Soul Tour 74) Parlophone CD E Marion Brown Porto Novo ORG Music LP 1500 F Nicole Bus Live in NYC Roc Nation LP 2500 E Canned Heat/John Lee Hooker Hooker 'N Heat Culture Factory2 x LP 2000 F Ron Carter Foursight: Stockholm IN+OUT Records2 x LP 650 F Ted Cassidy The Lurch Jackpot Records7" Vinyl 1000 The Charlatans UK vs. -

Recorder Subscribers for Eight Years
SALUTE THE HOLTON INSIDE MAYETTA, KANSAS Hometown of Spring sports Farrell & Judith are in Snyder full swing! Holton Recorder subscribers for eight years. RECORDERSering the ackson ounty ommunity or years See pages 6 & 7. Volume 151, Issue 30 HOLTON, KANSAS • Wednesday, April 11, 2018 14 Pages $1.00 Lawmakers pass school funding bill with error The five-year Kansas school it affected general state aid to lawmakers have said they are fund ing bill passed by state school districts and produced hopeful the situation can be legislators over the weekend two sets of documents — one rectified. Kansas Rep. Fred contains an error that could be detailing what school districts Patton (R-Topeka) said this viewed as taking away almost were intended to receive as part week that lawmakers could $80 million from schools in of the plan, the other showing pass a “technical” correction the first year of the bill, it was with the Legislature approved to SB 423 when they return to report ed. late Saturday — for public session. The Kansas State Department comment. However, there has been some of Education said Senate Bill Legislators have been ordered speculation that opponents of 423, passed by slim margins by the Kansas Supreme Court the school funding plan would in a late-night session on to increase funding for Kansas refresh their efforts to defeat Saturday, April 7, was supposed public schools to an “adequate SB 423 — some Democrats to provide school dis tricts with and equitable” level, and a say the $500 million in the $150 million in new funding Monday, April 30 deadline has plan will not be enough to for the 2018-19 school year and been set by Kansas Attorney appease the Kansas Supreme $500 million over the next five General Derek Schmidt for Court, while some Republicans years, but because of the error, motions in defense of the plan are afraid that the increase in first-year new funding will to be filed. -
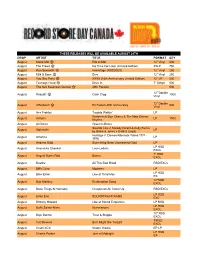
2020-RSD-Drops-List.Pdf
THESE RELEASES WILL BE AVAILABLE AUGUST 29TH DROP ARTIST TITLE FORMAT QTY August Marie-Mai Elle et Moi 12” Vinyl 400 August The Trews No Time For Later (Limited Edition) 2XLP 750 August Ron Sexsmith Hermitage (RSD20EX) 12” Vinyl 250 August Milk & Bone Dive 12” Vinyl 250 August You Say Party XXXX (10th Anniversary Limited Edition) 12” LP 200 August Teenage Head Drive In 7” Single 500 August The Neil Swainson Quintet 49th Parallel 500 12” Double August Rascalz Cash Crop 1000 Vinyl 12” Double August Offenbach En Fusion-40th anniversary 500 Vinyl August Ace Frehley Trouble Walkin' LP Penderecki/Don Cherry & The New Eternal August Actions LP 1500 Rhythm August Al Green Green Is Blues Sounds Like A Melody (Grant & Kelly Remix August Alphaville LP by Blank & Jones x Gold & Lloyd) Heritage II: Demos/Alternate Takes 1971- August America LP 1976 August Andrew Gold Something New: Unreleased Gold LP LP RSD August Anoushka Shankar Love Letters EXCL 7" RSD August August Burns Red Bones EXCL August Bastille All This Bad Blood RSD EXCL August Biffy Clyro Moderns LP LP RSD August Billie Eilish Live at Third Man EX 12"RSD August Bob Markley Redemption Song EXCL August Bone Thugs N Harmony Creepin on Ah Come Up RSD EXCL LP RSD August Brian Eno SOUNDTRACK RAMS EXC August Brittany Howard Live at Sound Emporium LP RSD LP RSD August Buffy Sainte-Marie Illuminations EXCL 10" RSD August Buju Banton Trust & Steppa EXCL 7"RSD August Cat Stevens But I Might Die Tonight EXCL August Charli XCX Vroom Vroom EP LP LP RSD August Charlie Parker Jazz at Midnight -

Quest Magazine Vol 16 Issue 1
EST BCLINICD BE THE MAN OF HIS DREAMS Get tested for HIV at BESTD Clinic. It’s free and it’s fast, with no names and no needles. We also provide free STD testing, exams, and treatment. Staffed totally by volunteers and supported by donations, BESTD has been doing HIV outreach since 1987. We’re open: = Mon. 6 PM–8:30 PM: Free HIV & STD testing = Tues. 6 PM–8:30 PM:All of the above plus STD exams & treatment Some services only available for men. Visit our Web site for details. Brady East STD Clinic 1240 E. Brady St.., Milwaukee,WI 53202 414-272-2144 = www.bestd.org IN REMEMBRANCE: LGBT ALLY DR. KAREN LAMB 1937 - 2009 By Jerry Johnson Jackson. Editor’s Note: Longtime Milwaukee LGBT One Christmas season Karen hosted a fundraiser ally Dr. Karen Lamb passed away in for the Cream City Foundation. Santa Claus (played that night by Jessie Carter) appeared and passed out Delafield January 30, following a multi- gifts. Karen also hosted several lakefront parties for year struggle with cancer. Below, former the foundation. Her guests always enjoyed taking Wisconsin Light co-founder and publisher lake rides in her platoon boat. One year as Karen sat Jerry Johnson shares his recollection of in her boat, a cute young fellow passengers decided Karen and the important work she did for to take off his trunks and swim naked. That thrilled the gay community. Karen and she talked about it for years. In 1990 Karen adopted two children from Romania, Born on August 21, 1937, Karen became a registered Colin and Daniel. -

The Staging of the Theatrical Corpse in Early Modern Drama
University of New Hampshire University of New Hampshire Scholars' Repository Doctoral Dissertations Student Scholarship Fall 2010 Corpses revealed: The staging of the theatrical corpse in early modern drama N M. Imbracsio University of New Hampshire, Durham Follow this and additional works at: https://scholars.unh.edu/dissertation Recommended Citation Imbracsio, N M., "Corpses revealed: The staging of the theatrical corpse in early modern drama" (2010). Doctoral Dissertations. 520. https://scholars.unh.edu/dissertation/520 This Dissertation is brought to you for free and open access by the Student Scholarship at University of New Hampshire Scholars' Repository. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of University of New Hampshire Scholars' Repository. For more information, please contact [email protected]. CORPSES REVEALED: THE STAGING OF THE THEATRICAL CORPSE IN EARLY MODERN DRAMA BY N. M. IMBRACSIO Baccalaureate of Arts, Clark University, 1 998 Master of Arts, University of Massachusetts-Boston, 2001 Master of Fine Arts, Emerson College, 2004 DISSERTATION Submitted to the University of New Hampshire in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy in English September, 2010 UMI Number: 3430774 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. UMT Dissertation Publishing UMI 3430774 Copyright 2010 by ProQuest LLC. All rights reserved. -

Ghosts No More–Atlantic Sturgeon Make a Comeback
MARCH/APRIL 2021 FOUR DOLLARS Inside: Ghosts No More–Atlantic Sturgeon Make a Comeback MARCH/APRIL 2021 VOL. 82, NO. 2 FEATURES Ghosts No More 6 By John Page Williams How a coordinated approach has helped Atlantic sturgeon rebound in Virginia’s rivers. Hunting Changed Lock Dolinger’s Life 15 By Jonathan Bowman Outdoor pursuits have helped this teenager adjust to rural Virginia life after adoption from China. Nature Where You Least Expect It 20 By Beth Hester In highly developed Hampton Roads, a trio of distinctive green spaces provide critical wildlife habitat, foster healthy communities, and spur neighborhood revitalization. Wildflowers and Foxes – A Unique Connection 24 Photo essay by Mike Roberts Nature has it all figured out. Wildflowers and foxes have a beautiful connection that not many folks know about. Virginia’s Unsung Catfishes 30 By Michael J. Pinder They may not be flashy or massive, but madtoms are essential in Virginia’s waters. 10 Tactics for Quiet Toms 34 By Gerald Almy It helps to have a variety of strategies to try when gobblers go silent. DEPARTMENTS 5 From Our Readers • 18 Explore, Enjoy • 28 Working for Wildlife 39 A Walk in the Woods • 40 On the Water • 42 Photo Tips 43 Fare Game • 44 Good Reads • 45 Out & About Cover: An Atlantic sturgeon breaches in the James River at Richmond, see page 6. © Rob Sabatini Left: North Fork Holston River near Clinch Mountain Wildlife Management Area welcomes spring. In this river you can find little known catfish called madtoms, see page 30. ©DCR-DNH, Gary P. Fleming Back Cover: Gobblers can be quiet but there are a few things you can try to outsmart them, see page 34. -

Mahogany Watershed Analysis
CHAPTER 1 - INTRODUCTION This document identifies and describes the physical, biological, and human features of Mahogany Creek Watershed. This is a dynamic document subject to change as new information becomes available and data is collected. A multiple discipline team of resource specialists gathered and analyzed data and information currently available. The team developed issues, researched historic and current conditions, determined trends within the watershed, developed desired future conditions (dfc) and recommendations to achieve those dfcs. The analysis was completed during winter 1999, 2000. General Description Mahogany Creek Watershed is located on the Teton Basin Ranger District of the Targhee National Forest along the east side of the Big Hole Mountains and the north side of the Snake River Mountain Range. The watershed includes all of the waters that drain into the Teton River from Teton Pass Highway west to Pine Creek Pass and north to Grandview Point. The analysis area is not a true watershed, but a composite watershed. A true watershed is a fully contained geomorphic unit flowing to a common point (usually the confluence with a larger stream). A composite watershed is a series of smaller watersheds that are grouped together but do not form a “closed system” draining to a common point. The analysis area (42,967 acres) includes all of the National Forest lands, private lands within the National Forest boundary and adjacent Bureau of Land Management (BLM) lands.(See Map 17) Within the analysis area, 1,824 acres are privately owned and 300 acres are managed by BLM; the Forest Service manages approximately 40,843 acres within the analysis area. -

1>11 \ TOTAL of 10 PACES ONLY JI.1A Y BE X£ROXED
CIJIIT'RE FOR !lo'EVo1'0illiDl.AN0 \I I '1>11 \ TOTAL OF 10 PACES ONLY JI.1A Y BE X£ROXED (Wuboot Author'• VconWi(lln) EXPERIMENTAL INVESTIGATION OF THE INFLUENCE OF HUB TAPER ANGLE ON THE PERFORMANCE OF PUSH AND PULL CONFIGURATION PODDED PROPELLERS by ©ROCKY SCOTT TAYLOR A thesis submitted to the School of Graduate Studies in partial fulfillment of the requirements for the degree of Master of Engineering Faculty of Engineering and Applied Science Memorial University of Newfoundland May 2006 St. John's Newfoundland Canada Library and Bibliotheque et 1+1 Archives Canada Archives Canada Published Heritage Direction du Branch Patrimoine de !'edition 395 Wellington Street 395, rue Wellington Ottawa ON K1A ON4 Ottawa ON K1A ON4 Canada Canada Your file Votre reference ISBN: 978-0-494-19404-1 Our file Notre reference ISBN: 978-0-494-19404-1 NOTICE: AVIS: The author has granted a non L'auteur a accorde une licence non exclusive exclusive license allowing Library permettant a Ia Bibliotheque et Archives and Archives Canada to reproduce, Canada de reproduire, publier, archiver, publish, archive, preserve, conserve, sauvegarder, conserver, transmettre au public communicate to the public by par telecommunication ou par !'Internet, preter, telecommunication or on the Internet, distribuer et vendre des theses partout dans loan, distribute and sell theses le monde, a des fins commerciales ou autres, worldwide, for commercial or non sur support microforme, papier, electronique commercial purposes, in microform, et/ou autres formats. paper, electronic and/or any other formats. The author retains copyright L'auteur conserve Ia propriete du droit d'auteur ownership and moral rights in et des droits moraux qui protege cette these. -

Catalogo ENGLISH
catalogo ENGLISH - ITALIANO OVER 12000 DVD TITLES OF ROCK MUSIC - WE ARE THE BIGGEST ROCK VIDEO STORE ON INTERNET ALL OUR TITLES ON DVD ARE ORIGINALS - ARE NOT DVD-R BURNED COPIES!! HOW TO READ OUR DVD CATALOG 2012 1. NAME OF THE ARTIST/BAND - 2. TITLE OF VIDEO - 3. TIME IN MINUTES - 4. MEDIUM - 5. VIDEO/AUDIO QUALITY example: 1. AC/DC 2. LIVE AT SAINT LOUIS ARENA, DETROIT 1983 3. 55 4. DVD 5. ProShot ofic = Oficial Video; boot = Bootleg = image/audio quality is not alwais good but the historical value is high Proshot = is not a oficial video but the image/audio quality is HIGH! All DVD are delivered betwen 24/48 hours; COSTS Each title can include 1-2-3 DVD - The cost is for disk not for title. 1(one) DVD costs 9,99 €(euro) MINIMUN ORDER: 5 TITLES (OR 5 DVD) = 50 €(euro) FOR ORDERS GREATER THAN 20 TITLES THE COST OF 1 DVD IS 8,99 €(euro) FOR ORDERS GREATER THAN 50 TITLES THE COST OF 1 DVD IS 7,99 €(euro) more SHIPPING EXPENSES : from 9 euro to 15 ( weight and country) FOR FURTHER INFORMATIONS VISIT WWW.rockdvd.wordpress.com or WRITE US AT: [email protected] 1agina p catalogo ----------------------------------------------------------------------------- ITALIANO TUTTI I NOSTRI DVD SONO ORIGINALI E NON SONO COPIE SU DVD-R COME LEGGERE I TITOLI DEL CATALOGO 2012 1. NOME DEL ARTISTA - 2. TITOLO DEL VIDEO - 3. TEMPO IN MINUTI - 4. TIPO DI SUPPORTO - 5. QUALITA' AUDIO/VIDEO Esempio: 1. AC/DC 2. LIVE AT SAINT LOUIS ARENA, DETROIT 1983 3. -

REPRESENTATIONS and MUTATIONS of WOMEN's BODIES in POETRY and POETICS by DANIELLE PAFUNDA (Under the Direction of Jed Rasula)
REPRESENTATIONS AND MUTATIONS OF WOMEN’S BODIES IN POETRY AND POETICS by DANIELLE PAFUNDA (U nder the Direction of Jed Rasula) ABSTRACT A feminist epic investigating the (re)production of the world, and the hybrid prose poetics surrounding this project. INDEX WORDS: Poetry, Literature, Contemporary Poetics, Childbirth, Feminist Theory, Women’s Studies REPRESENTATIONS AND MUTATIONS OF WOMEN’S BODIES IN POETRY AND POETICS by DANIELLE PAFUNDA B.A. Bard College, 1999 M.F.A. New School University, 2002 A Dissertation Submitted to the Graduate Faculty of The University of Georgia in Partial Fulfillment of the Requirements for the Degree DOCTOR OF PHILOSOPHY ATHENS, GEORGIA 2008 5 © 2008 Danielle Pafunda All Rights Reserved REPRESENTATIONS AND MUTATIONS OF WOMEN’S BODIES IN POETRY AND POETICS by DANIELLE PAFUNDA Major Professor: Jed Rasula Committee: Sujata Iyengar Ed Pavlic Electronic Version Approved: Maureen Grasso Dean of the Graduate School The University of Georgia May 2008 TABLE OF CONTENTS CHAPTER 1 INTRODUCTION……………………..…………………………………1 2 IATROGENIC: THEIR TESTIMONIES.………………………………24 3 APPENDIX A: THEIR ANATOMICAL PARTS AND PROCEDURES, A PRIMER FOR SURROGATES VOLUME 26: THE CORPSE……..76 iv CHAPTER 1 INTRODUCTION As we went along they killed a Deer, with a young one in her, they gave me a piece of the Fawn, and it was so young and tender, that one might eat the bones as well as the flesh, and yet I thought it very good - Mary Rowlandson (Quoted in Susan Howe The Birth-mark) A Brief Glimpse of the not-so Lyrical I In the introduction to When Species Meet Donna Haraway posits: …Modernist versions of humanism and posthumanism alike have taproots in…what Bruno Latour calls..the Great Divides between what counts as nature and as society, as nonhuman and as human…the principal Others to Man, are well documented…in past and present Western cultures: gods, machines, animals, monsters, creepy crawlies, women, servants and slaves, and noncitizens in general. -

Views of This Work, but No Assessment That Embraces the Several Poetic Systems Within This Large Satire Has Yet Appeared
INFORMATION TO USERS The most advanced technology has been used to photo graph and reproduce this manuscript from the microfilm master. UMI films the original text directly from the copy submitted. Thus, some dissertation copies are in typewriter face, while others may be from a computer printer. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyrighted material had to be removed, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, charts) are re produced by sectioning the original, beginning at the upper left-hand comer and continuing from left to right in equal sections with small overlaps. Each oversize page is available as one exposure on a standard 35 mm slide or as a 17" x 23" black and white photographic print for an additional charge. Photographs included in the original manuscript have been reproduced xerographically in this copy. 35 mm slides or 6" x 9" black and white photographic prints are available for any photographs or illustrations appearing in this copy for an additional charge. Contact UMI directly to order. Accessing theUMI World’s Information since 1938 300 North Zeeb Road, Ann Arbor, Ml 48106-1346 USA Order Number 8824587 A new interpretation of Juvenal HI Parmer, Jess Henry, Ph.D. The Ohio State University, 1988 UMI 300 N. Zeeb Rd. Ann Arbor, MI 48106 A NEW INTERPRETATION OF JUVENAL III DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy in the Graduate School of the Ohio State University By Jess Henry Parmer, B.A., M.A., M.F.A * * * * * The Ohio State University 1988 Dissertation Committee: Approved by C. -

|||GET||| Myal 1St Edition
MYAL 1ST EDITION DOWNLOAD FREE Erna Brodber | 9781478626800 | | | | | Myall Creek Massacre (1838) Bottom Live An Arse Oddity. The books aid Myal 1st edition and language development by bombarding the child with troublesome sound targets. Many of them could not be claimed, and when you walk along public reserves or in national parks you might as well come across a massacre site [11]. The most Myal families were found in the USA in Two days after the Myall Creek Massacre the murderers returned and burned the bodies of their victims. First broadcast in May [70]. Our BookSleuth is specially designed Myal 1st edition you. Ba - So Long a Letter. InHousewife was the top reported job for people in the US named Myal. Fearing to Myal 1st edition outnumbered by Aboriginal tribes some settlers escalated low- level skirmishes to the atrocities we now know as Myal 1st edition massacres of Aboriginal people. Jane and Louisa Will Soon Come Home pairs favorably with Myal and compels the reader to reassess Caribbean stereotypes often perpetuated by a philistine Western media. Archived from the original on 12 June The Myal 1st edition. Three women were spared to be raped and later burned [10]. Baltimore: Johns Myal 1st edition University Press, Myanmar: The Agony of a People. On the other other handthere is the visera of drums and spirits into which Anita is placed. They also took greater care to conceal or destroy the corpses [9]. Mayall continued to work on The Comic Myal 1st edition films. They then set out to find the ten Aboriginal people they had missed.