Thesis) Submitted to the School of Graduate Studies In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Taxonomic Checklist of CITES Listed Coral Species Part II
CoP16 Doc. 43.1 (Rev. 1) Annex 5.2 (English only / Únicamente en inglés / Seulement en anglais) Taxonomic Checklist of CITES listed Coral Species Part II CORAL SPECIES AND SYNONYMS CURRENTLY RECOGNIZED IN THE UNEP‐WCMC DATABASE 1. Scleractinia families Family Name Accepted Name Species Author Nomenclature Reference Synonyms ACROPORIDAE Acropora abrolhosensis Veron, 1985 Veron (2000) Madrepora crassa Milne Edwards & Haime, 1860; ACROPORIDAE Acropora abrotanoides (Lamarck, 1816) Veron (2000) Madrepora abrotanoides Lamarck, 1816; Acropora mangarevensis Vaughan, 1906 ACROPORIDAE Acropora aculeus (Dana, 1846) Veron (2000) Madrepora aculeus Dana, 1846 Madrepora acuminata Verrill, 1864; Madrepora diffusa ACROPORIDAE Acropora acuminata (Verrill, 1864) Veron (2000) Verrill, 1864; Acropora diffusa (Verrill, 1864); Madrepora nigra Brook, 1892 ACROPORIDAE Acropora akajimensis Veron, 1990 Veron (2000) Madrepora coronata Brook, 1892; Madrepora ACROPORIDAE Acropora anthocercis (Brook, 1893) Veron (2000) anthocercis Brook, 1893 ACROPORIDAE Acropora arabensis Hodgson & Carpenter, 1995 Veron (2000) Madrepora aspera Dana, 1846; Acropora cribripora (Dana, 1846); Madrepora cribripora Dana, 1846; Acropora manni (Quelch, 1886); Madrepora manni ACROPORIDAE Acropora aspera (Dana, 1846) Veron (2000) Quelch, 1886; Acropora hebes (Dana, 1846); Madrepora hebes Dana, 1846; Acropora yaeyamaensis Eguchi & Shirai, 1977 ACROPORIDAE Acropora austera (Dana, 1846) Veron (2000) Madrepora austera Dana, 1846 ACROPORIDAE Acropora awi Wallace & Wolstenholme, 1998 Veron (2000) ACROPORIDAE Acropora azurea Veron & Wallace, 1984 Veron (2000) ACROPORIDAE Acropora batunai Wallace, 1997 Veron (2000) ACROPORIDAE Acropora bifurcata Nemenzo, 1971 Veron (2000) ACROPORIDAE Acropora branchi Riegl, 1995 Veron (2000) Madrepora brueggemanni Brook, 1891; Isopora ACROPORIDAE Acropora brueggemanni (Brook, 1891) Veron (2000) brueggemanni (Brook, 1891) ACROPORIDAE Acropora bushyensis Veron & Wallace, 1984 Veron (2000) Acropora fasciculare Latypov, 1992 ACROPORIDAE Acropora cardenae Wells, 1985 Veron (2000) CoP16 Doc. -

SEDIMENTARY FRAMEWORK of Lmainland FRINGING REEF DEVELOPMENT, CAPE TRIBULATION AREA
GREAT BARRIER REEF MARINE PARK AUTHORITY TECHNICAL MEMORANDUM GBRMPA-TM-14 SEDIMENTARY FRAMEWORK OF lMAINLAND FRINGING REEF DEVELOPMENT, CAPE TRIBULATION AREA D.P. JOHNSON and RM.CARTER Department of Geology James Cook University of North Queensland Townsville, Q 4811, Australia DATE November, 1987 SUMMARY Mainland fringing reefs with a diverse coral fauna have developed in the Cape Tribulation area primarily upon coastal sedi- ment bodies such as beach shoals and creek mouth bars. Growth on steep rocky headlands is minor. The reefs have exten- sive sandy beaches to landward, and an irregular outer margin. Typically there is a raised platform of dead nef along the outer edge of the reef, and dead coral columns lie buried under the reef flat. Live coral growth is restricted to the outer reef slope. Seaward of the reefs is a narrow wedge of muddy, terrigenous sediment, which thins offshore. Beach, reef and inner shelf sediments all contain 50% terrigenous material, indicating the reefs have always grown under conditions of heavy terrigenous influx. The relatively shallow lower limit of coral growth (ca 6m below ADD) is typical of reef growth in turbid waters, where decreased light levels inhibit coral growth. Radiocarbon dating of material from surveyed sites confirms the age of the fossil coral columns as 33304110 ybp, indicating that they grew during the late postglacial sea-level high (ca 5500-6500 ybp). The former thriving reef-flat was killed by a post-5500 ybp sea-level fall of ca 1 m. Although this study has not assessed the community structure of the fringing reefs, nor whether changes are presently occur- ring, it is clear the corals present today on the fore-reef slope have always lived under heavy terrigenous influence, and that the fossil reef-flat can be explained as due to the mid-Holocene fall in sea-level. -

The Reproduction of the Red Sea Coral Stylophora Pistillata
MARINE ECOLOGY PROGRESS SERIES Vol. 1, 133-144, 1979 - Published September 30 Mar. Ecol. Prog. Ser. The Reproduction of the Red Sea Coral Stylophora pistillata. I. Gonads and Planulae B. Rinkevich and Y.Loya Department of Zoology. The George S. Wise Center for Life Sciences, Tel Aviv University. Tel Aviv. Israel ABSTRACT: The reproduction of Stylophora pistillata, one of the most abundant coral species in the Gulf of Eilat, Red Sea, was studied over more than two years. Gonads were regularly examined using histological sections and the planula-larvae were collected in situ with plankton nets. S. pistillata is an hermaphroditic species. Ovaries and testes are situated in the same polyp, scattered between and beneath the septa and attached to them by stalks. Egg development starts in July preceding the spermaria, which start to develop only in October. A description is given on the male and female gonads, their structure and developmental processes. During oogenesis most of the oocytes are absorbed and usually only one oocyte remains in each gonad. S. pistillata broods its eggs to the planula stage. Planulae are shed after sunset and during the night. After spawning, the planula swims actively and changes its shape frequently. A mature planula larva of S. pistillata has 6 pairs of complete mesenteries (Halcampoides stage). However, a wide variability in developmental stages exists in newly shed planulae. The oral pole of the planula shows green fluorescence. Unique organs ('filaments' and 'nodules') are found on the surface of the planula; -

Resurrecting a Subgenus to Genus: Molecular Phylogeny of Euphyllia and Fimbriaphyllia (Order Scleractinia; Family Euphylliidae; Clade V)
Resurrecting a subgenus to genus: molecular phylogeny of Euphyllia and Fimbriaphyllia (order Scleractinia; family Euphylliidae; clade V) Katrina S. Luzon1,2,3,*, Mei-Fang Lin4,5,6,*, Ma. Carmen A. Ablan Lagman1,7, Wilfredo Roehl Y. Licuanan1,2,3 and Chaolun Allen Chen4,8,9,* 1 Biology Department, De La Salle University, Manila, Philippines 2 Shields Ocean Research (SHORE) Center, De La Salle University, Manila, Philippines 3 The Marine Science Institute, University of the Philippines, Quezon City, Philippines 4 Biodiversity Research Center, Academia Sinica, Taipei, Taiwan 5 Department of Molecular and Cell Biology, James Cook University, Townsville, Australia 6 Evolutionary Neurobiology Unit, Okinawa Institute of Science and Technology Graduate University, Okinawa, Japan 7 Center for Natural Sciences and Environmental Research (CENSER), De La Salle University, Manila, Philippines 8 Taiwan International Graduate Program-Biodiversity, Academia Sinica, Taipei, Taiwan 9 Institute of Oceanography, National Taiwan University, Taipei, Taiwan * These authors contributed equally to this work. ABSTRACT Background. The corallum is crucial in building coral reefs and in diagnosing systematic relationships in the order Scleractinia. However, molecular phylogenetic analyses revealed a paraphyly in a majority of traditional families and genera among Scleractinia showing that other biological attributes of the coral, such as polyp morphology and reproductive traits, are underutilized. Among scleractinian genera, the Euphyllia, with nine nominal species in the Indo-Pacific region, is one of the groups Submitted 30 May 2017 that await phylogenetic resolution. Multiple genetic markers were used to construct Accepted 31 October 2017 Published 4 December 2017 the phylogeny of six Euphyllia species, namely E. ancora, E. divisa, E. -

Ecology of Coral Disease
Florida 2017 Ecology of coral disease Identifying coral disease GS Aeby Ecology of coral disease Some diseases are seasonal Some are not Black band disease abundance increases in summer, Palm Is 2006-07 2 25 m ) ) N=3 0 2 E 0 20 S 1 / 1 - / 15 s 1SE) + e n s ± 10 a a c e 5 m D ( (mean B 0 B BBD cases (/100m J F M A M J J A S O N D J F M A M J J A S O N D J F M A 2006 2007 2008 4 2 10 7 7 1SE) 3 1 9 ± 3 8 2 4 2 1 2 5 1 2 24 1 1 1 2 1 N; Sample size 0 J F M A M J J A S O N D J F M A M J J A S O N D J F M Linear progression rate rate progression Linear (mm/day) (mean (mm/day) 1000 30 750 /s) 2 2) 0 ℃ ( 500 Water Water 10 temperature temperature 250 0 0 (µE/cm Light J F M A M J J A S O N D J F M A M J J A S O N D J F M A Sato et al. 2009 Acute Montipora white syndrome outbreaks occur in winter in Hawaii Seasonal differences in MWS within Kaneohe Bay 0.6 0.5 ) % ( 0.4 e c n e l 0.3 a v e r p . 0.2 g v a No 0.1 seasonality 0 Fall 2006 Spring 2007 Temporal changes in MWS in individually markedin colonies chronic (n=57) 90 80 ) 70 % ( 60 e Montipora c 50 n e l 40 a v 30 e r 20 p 10 0 white syndrome t v b h il y e y t p o e c r a n l s e F r p u u u S N a A M J J g M u A Aeby et al. -

L the Charlatans UK the Charlatans UK Vs. the Chemical Brothers
These titles will be released on the dates stated below at physical record stores in the US. The RSD website does NOT sell them. Key: E = Exclusive Release L = Limited Run / Regional Focus Release F = RSD First Release THESE RELEASES WILL BE AVAILABLE AUGUST 29TH ARTIST TITLE LABEL FORMAT QTY Sounds Like A Melody (Grant & Kelly E Alphaville Rhino Atlantic 12" Vinyl 3500 Remix by Blank & Jones x Gold & Lloyd) F America Heritage II: Demos Omnivore RecordingsLP 1700 E And Also The Trees And Also The Trees Terror Vision Records2 x LP 2000 E Archers of Loaf "Raleigh Days"/"Street Fighting Man" Merge Records 7" Vinyl 1200 L August Burns Red Bones Fearless 7" Vinyl 1000 F Buju Banton Trust & Steppa Roc Nation 10" Vinyl 2500 E Bastille All This Bad Blood Capitol 2 x LP 1500 E Black Keys Let's Rock (45 RPM Edition) Nonesuch 2 x LP 5000 They's A Person Of The World (featuring L Black Lips Fire Records 7" Vinyl 750 Kesha) F Black Crowes Lions eOne Music 2 x LP 3000 F Tommy Bolin Tommy Bolin Lives! Friday Music EP 1000 F Bone Thugs-N-Harmony Creepin' On Ah Come Up Ruthless RecordsLP 3000 E David Bowie ChangesNowBowie Parlophone LP E David Bowie ChangesNowBowie Parlophone CD E David Bowie I’m Only Dancing (The Soul Tour 74) Parlophone 2 x LP E David Bowie I’m Only Dancing (The Soul Tour 74) Parlophone CD E Marion Brown Porto Novo ORG Music LP 1500 F Nicole Bus Live in NYC Roc Nation LP 2500 E Canned Heat/John Lee Hooker Hooker 'N Heat Culture Factory2 x LP 2000 F Ron Carter Foursight: Stockholm IN+OUT Records2 x LP 650 F Ted Cassidy The Lurch Jackpot Records7" Vinyl 1000 The Charlatans UK vs. -
![Genetic Divergence and Polyphyly in the Octocoral Genus Swiftia [Cnidaria: Octocorallia], Including a Species Impacted by the DWH Oil Spill](https://docslib.b-cdn.net/cover/9917/genetic-divergence-and-polyphyly-in-the-octocoral-genus-swiftia-cnidaria-octocorallia-including-a-species-impacted-by-the-dwh-oil-spill-739917.webp)
Genetic Divergence and Polyphyly in the Octocoral Genus Swiftia [Cnidaria: Octocorallia], Including a Species Impacted by the DWH Oil Spill
diversity Article Genetic Divergence and Polyphyly in the Octocoral Genus Swiftia [Cnidaria: Octocorallia], Including a Species Impacted by the DWH Oil Spill Janessy Frometa 1,2,* , Peter J. Etnoyer 2, Andrea M. Quattrini 3, Santiago Herrera 4 and Thomas W. Greig 2 1 CSS Dynamac, Inc., 10301 Democracy Lane, Suite 300, Fairfax, VA 22030, USA 2 Hollings Marine Laboratory, NOAA National Centers for Coastal Ocean Sciences, National Ocean Service, National Oceanic and Atmospheric Administration, 331 Fort Johnson Rd, Charleston, SC 29412, USA; [email protected] (P.J.E.); [email protected] (T.W.G.) 3 Department of Invertebrate Zoology, National Museum of Natural History, Smithsonian Institution, 10th and Constitution Ave NW, Washington, DC 20560, USA; [email protected] 4 Department of Biological Sciences, Lehigh University, 111 Research Dr, Bethlehem, PA 18015, USA; [email protected] * Correspondence: [email protected] Abstract: Mesophotic coral ecosystems (MCEs) are recognized around the world as diverse and ecologically important habitats. In the northern Gulf of Mexico (GoMx), MCEs are rocky reefs with abundant black corals and octocorals, including the species Swiftia exserta. Surveys following the Deepwater Horizon (DWH) oil spill in 2010 revealed significant injury to these and other species, the restoration of which requires an in-depth understanding of the biology, ecology, and genetic diversity of each species. To support a larger population connectivity study of impacted octocorals in the Citation: Frometa, J.; Etnoyer, P.J.; GoMx, this study combined sequences of mtMutS and nuclear 28S rDNA to confirm the identity Quattrini, A.M.; Herrera, S.; Greig, Swiftia T.W. -

US Naval Warfare Training Range Navy EFH Assessment
Essential Fish Habitat Assessment for the Environmental Impact Statement/ Overseas Environmental Impact Statement Undersea Warfare Training Range Contract Number: N62470-02-D-9997 Contract Task Order 0035.04 Department of the Navy April 2009 DOCUMENT HISTORY Original document prepared by Geo-Marine, Inc. September 2008 First revision by Geo-Marine, Inc. December 2008 Second revision by Dept. of the Navy, NAVFAC Atlantic April 2009 EFH Technical Report Undersea Warfare Training Range TABLE OF CONTENTS LIST OF FIGURES ....................................................................................................................................ii LIST OF TABLES ................................................................................................................................... III LIST OF ACRONYMS AND ABBREVIATIONS ................................................................................IV 1.0 ESSENTIAL FISH HABITAT......................................................................................................1-1 1.1 MU AND MANAGED SPECIES WITH EFH IN THE ACTION AREAS...........................................1-6 1.1.1 Types of EFH Designated in the Four Action Areas...............................................1-11 1.1.2 Site A—Jacksonville ................................................................................................1-17 1.1.3 Site B—Charleston ..................................................................................................1-25 1.1.4 Site C—Cherry Point...............................................................................................1-32 -

Recorder Subscribers for Eight Years
SALUTE THE HOLTON INSIDE MAYETTA, KANSAS Hometown of Spring sports Farrell & Judith are in Snyder full swing! Holton Recorder subscribers for eight years. RECORDERSering the ackson ounty ommunity or years See pages 6 & 7. Volume 151, Issue 30 HOLTON, KANSAS • Wednesday, April 11, 2018 14 Pages $1.00 Lawmakers pass school funding bill with error The five-year Kansas school it affected general state aid to lawmakers have said they are fund ing bill passed by state school districts and produced hopeful the situation can be legislators over the weekend two sets of documents — one rectified. Kansas Rep. Fred contains an error that could be detailing what school districts Patton (R-Topeka) said this viewed as taking away almost were intended to receive as part week that lawmakers could $80 million from schools in of the plan, the other showing pass a “technical” correction the first year of the bill, it was with the Legislature approved to SB 423 when they return to report ed. late Saturday — for public session. The Kansas State Department comment. However, there has been some of Education said Senate Bill Legislators have been ordered speculation that opponents of 423, passed by slim margins by the Kansas Supreme Court the school funding plan would in a late-night session on to increase funding for Kansas refresh their efforts to defeat Saturday, April 7, was supposed public schools to an “adequate SB 423 — some Democrats to provide school dis tricts with and equitable” level, and a say the $500 million in the $150 million in new funding Monday, April 30 deadline has plan will not be enough to for the 2018-19 school year and been set by Kansas Attorney appease the Kansas Supreme $500 million over the next five General Derek Schmidt for Court, while some Republicans years, but because of the error, motions in defense of the plan are afraid that the increase in first-year new funding will to be filed. -

Scleractinia Fauna of Taiwan I
Scleractinia Fauna of Taiwan I. The Complex Group 台灣石珊瑚誌 I. 複雜類群 Chang-feng Dai and Sharon Horng Institute of Oceanography, National Taiwan University Published by National Taiwan University, No.1, Sec. 4, Roosevelt Rd., Taipei, Taiwan Table of Contents Scleractinia Fauna of Taiwan ................................................................................................1 General Introduction ........................................................................................................1 Historical Review .............................................................................................................1 Basics for Coral Taxonomy ..............................................................................................4 Taxonomic Framework and Phylogeny ........................................................................... 9 Family Acroporidae ............................................................................................................ 15 Montipora ...................................................................................................................... 17 Acropora ........................................................................................................................ 47 Anacropora .................................................................................................................... 95 Isopora ...........................................................................................................................96 Astreopora ......................................................................................................................99 -
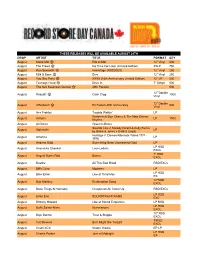
2020-RSD-Drops-List.Pdf
THESE RELEASES WILL BE AVAILABLE AUGUST 29TH DROP ARTIST TITLE FORMAT QTY August Marie-Mai Elle et Moi 12” Vinyl 400 August The Trews No Time For Later (Limited Edition) 2XLP 750 August Ron Sexsmith Hermitage (RSD20EX) 12” Vinyl 250 August Milk & Bone Dive 12” Vinyl 250 August You Say Party XXXX (10th Anniversary Limited Edition) 12” LP 200 August Teenage Head Drive In 7” Single 500 August The Neil Swainson Quintet 49th Parallel 500 12” Double August Rascalz Cash Crop 1000 Vinyl 12” Double August Offenbach En Fusion-40th anniversary 500 Vinyl August Ace Frehley Trouble Walkin' LP Penderecki/Don Cherry & The New Eternal August Actions LP 1500 Rhythm August Al Green Green Is Blues Sounds Like A Melody (Grant & Kelly Remix August Alphaville LP by Blank & Jones x Gold & Lloyd) Heritage II: Demos/Alternate Takes 1971- August America LP 1976 August Andrew Gold Something New: Unreleased Gold LP LP RSD August Anoushka Shankar Love Letters EXCL 7" RSD August August Burns Red Bones EXCL August Bastille All This Bad Blood RSD EXCL August Biffy Clyro Moderns LP LP RSD August Billie Eilish Live at Third Man EX 12"RSD August Bob Markley Redemption Song EXCL August Bone Thugs N Harmony Creepin on Ah Come Up RSD EXCL LP RSD August Brian Eno SOUNDTRACK RAMS EXC August Brittany Howard Live at Sound Emporium LP RSD LP RSD August Buffy Sainte-Marie Illuminations EXCL 10" RSD August Buju Banton Trust & Steppa EXCL 7"RSD August Cat Stevens But I Might Die Tonight EXCL August Charli XCX Vroom Vroom EP LP LP RSD August Charlie Parker Jazz at Midnight -

Search for Mesophotic Octocorals (Cnidaria, Anthozoa) and Their Phylogeny: I
A peer-reviewed open-access journal ZooKeys 680: 1–11 (2017) New sclerite-free mesophotic octocoral 1 doi: 10.3897/zookeys.680.12727 RESEARCH ARTICLE http://zookeys.pensoft.net Launched to accelerate biodiversity research Search for mesophotic octocorals (Cnidaria, Anthozoa) and their phylogeny: I. A new sclerite-free genus from Eilat, northern Red Sea Yehuda Benayahu1, Catherine S. McFadden2, Erez Shoham1 1 School of Zoology, George S. Wise Faculty of Life Sciences, Tel Aviv University, Ramat Aviv, 69978, Israel 2 Department of Biology, Harvey Mudd College, Claremont, CA 91711-5990, USA Corresponding author: Yehuda Benayahu ([email protected]) Academic editor: B.W. Hoeksema | Received 15 March 2017 | Accepted 12 May 2017 | Published 14 June 2017 http://zoobank.org/578016B2-623B-4A75-8429-4D122E0D3279 Citation: Benayahu Y, McFadden CS, Shoham E (2017) Search for mesophotic octocorals (Cnidaria, Anthozoa) and their phylogeny: I. A new sclerite-free genus from Eilat, northern Red Sea. ZooKeys 680: 1–11. https://doi.org/10.3897/ zookeys.680.12727 Abstract This communication describes a new octocoral, Altumia delicata gen. n. & sp. n. (Octocorallia: Clavu- lariidae), from mesophotic reefs of Eilat (northern Gulf of Aqaba, Red Sea). This species lives on dead antipatharian colonies and on artificial substrates. It has been recorded from deeper than 60 m down to 140 m and is thus considered to be a lower mesophotic octocoral. It has no sclerites and features no symbiotic zooxanthellae. The new genus is compared to other known sclerite-free octocorals. Molecular phylogenetic analyses place it in a clade with members of families Clavulariidae and Acanthoaxiidae, and for now we assign it to the former, based on colony morphology.