Chemistry Connections Sample Questions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Multivalent Metals and Polyatomic Ions 1
Name Date Comprehension Section 4.2 Use with textbook pages 189–193. Multivalent metals and polyatomic ions 1. Define the following terms: (a) ionic compound (b) multivalent metal (c) polyatomic ion 2. Write the formulae and names of the compounds with the following combination of ions. The first row is completed to help guide you. Positive ion Negative ion Formula Compound name (a) Pb2+ O2– PbO lead(II) oxide (b) Sb4+ S2– (c) TlCl (d) tin(II) fluoride (e) Mo2S3 (f) Rh4+ Br– (g) copper(I) telluride (h) NbI5 (i) Pd2+ Cl– 3. Write the chemical formula for each of the following compounds. (a) manganese(II) chloride (f) vanadium(V) oxide (b) chromium(III) sulphide (g) rhenium(VII) arsenide (c) titanium(IV) oxide (h) platinum(IV) nitride (d) uranium(VI) fluoride (i) nickel(II) cyanide (e) nickel(II) sulphide (j) bismuth(V) phosphide 68 MHR • Section 4.2 Names and Formulas of Compounds © 2008 McGraw-Hill Ryerson Limited 0056_080_BCSci10_U2CH04_098461.in6856_080_BCSci10_U2CH04_098461.in68 6688 PDF Pass 77/11/08/11/08 55:25:38:25:38 PPMM Name Date Comprehension Section 4.2 4. Write the formulae for the compounds formed from the following ions. Then name the compounds. Ions Formula Compound name + – (a) K NO3 KNO3 potassium nitrate 2+ 2– (b) Ca CO3 + – (c) Li HSO4 2+ 2– (d) Mg SO3 2+ – (e) Sr CH3COO + 2– (f) NH4 Cr2O7 + – (g) Na MnO4 + – (h) Ag ClO3 (i) Cs+ OH– 2+ 2– (j) Ba CrO4 5. Write the chemical formula for each of the following compounds. (a) barium bisulphate (f) calcium phosphate (b) sodium chlorate (g) aluminum sulphate (c) potassium chromate (h) cadmium carbonate (d) calcium cyanide (i) silver nitrite (e) potassium hydroxide (j) ammonium hydrogen carbonate © 2008 McGraw-Hill Ryerson Limited Section 4.2 Names and Formulas of Compounds • MHR 69 0056_080_BCSci10_U2CH04_098461.in6956_080_BCSci10_U2CH04_098461.in69 6699 PDF Pass77/11/08/11/08 55:25:39:25:39 PPMM Name Date Comprehension Section 4.2 Use with textbook pages 186–196. -

CHEM 1411 Nomenclature Homework - Answers Part I
1 CHEM 1411 Nomenclature Homework - Answers Part I 1. The following are a list of binary and pseudobinary ionic compounds. Write the name when the formula is given. Write the formula when the name is given. (a) AlCl3 aluminum chloride (k) rubidium oxide Rb2O (b) AuBr3 gold (III) bromide (l) chromium (III) selenide Cr2Se3 (c) Na2S sodium sulfide (m) barium iodide BaI2 (d) Cu3P2 copper (II) phosphide (n) copper (I) fluoride CuF (e) Fe(OH)2 iron (II) hydroxide (o) copper (II) fluoride CuF2 (f) NH4OH ammonium hydroxide (p) strontium cyanide Sr(CN)2 (g) Co(CH3COO)3 cobalt (III) acetate (q) mercury (II) bromide HgBr2 (h) Zn(SCN)2 zinc thiocyanate (r) mercury (I) bromide Hg2Br2 (i) CaCrO4 calcium chromate (s) magnesium permanganate Mg(MnO4)2 (j) K2Cr2O7 potassium dichromate (t) lithium nitride Li3N 2. The following are lists of covalent compounds. Write the name when a formula is given. Write the formula when given a name. (a) CSe2 carbon diselenide (h) dichlorine heptoxide Cl2O7 (b) SF6 sulfur hexafluoride (i) xenon tetrafluoride XeF4 (c) BrF5 bromine pentafluoride (j) carbon monoxide CO (d) P4O10 tetraphosphorous decoxide (k) oxygen O2 (e) Cl2O dichlorine oxide (l) diboron trioxide B2B O3 (f) NH3 ammonia (m) arsenic trifluoride AsF3 (g) N2 dinitrogen or nitrogen (n) diiodine I2 2 3. The following are lists of acids or acid-forming compounds. Write the name when the formula is given. Write the formula when the name is given. (a) H3PO2 hypophosphorous acid (k) hydrogen cyanide HCN (g) (b) H2SO4 sulfuric acid (l) periodic acid HIO4 (c) HClO hypochlorous acid (m) hypochlorous acid HClO (d) H3PO4 phosphoric acid (n) nitric acid HNO3 (e) HBrO4 perbromic acid (o) acetic acid CH3CO2H (f) HIO2 iodous acid (p) chloric acid HClO3 (g) HI (g) hydrogen iodide (q) perbromic acid HBrO4 (h) HI (aq) hydroiodic acid (r) hydrofluoric acid HF (aq) (i) HCN (aq) hydrocyanic acid (s) phosphorous acid H3PO3 (j) HBrO hypobromous acid (t) hydrosulfuric acid H2S (aq) 4. -

Chlorine Dioxide Gas Treatment of Cantaloupe and Residue Analysis Simran Kaur Purdue University
Purdue University Purdue e-Pubs Open Access Theses Theses and Dissertations 2013 Chlorine Dioxide Gas Treatment of Cantaloupe and Residue Analysis Simran Kaur Purdue University Follow this and additional works at: https://docs.lib.purdue.edu/open_access_theses Part of the Food Science Commons Recommended Citation Kaur, Simran, "Chlorine Dioxide Gas Treatment of Cantaloupe and Residue Analysis" (2013). Open Access Theses. 47. https://docs.lib.purdue.edu/open_access_theses/47 This document has been made available through Purdue e-Pubs, a service of the Purdue University Libraries. Please contact [email protected] for additional information. CHLORINE DIOXIDE GAS TREATMENT OF CANTALOUPE AND RESIDUE ANALYSIS A Thesis Submitted to the Faculty of Purdue University by Simran Kaur In Partial Fulfillment of the Requirements for the Degree of Master of Science December 2013 Purdue University West Lafayette, Indiana ii Two cantaloupes were in love. One said to the other, “Let’s run away together!” The other one said, “No. We cantelope.” iii ACKNOWLEDGEMENTS I would like to thank Dr. Mark Morgan for giving me the opportunity to work on this project. I truly appreciate his support, encouragement and guidance and have thoroughly enjoyed every moment. I would also like to thank my committee members, Dr. Mario Ferruzzi and Dr. Peter Hirst for their suggestions, feedback and comments. Thank you to Ben Paxson for his help with equipment and also to Dr. Applegate for the lab space to do my project. I am also thankful for the help and support from all my friends, old and new, close and far, for their support and companionship throughout my graduate studies, especially my dear friend Nadra Guizani. -
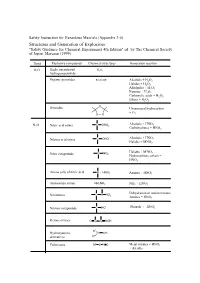
Appendix 3-4 Structures and Generation of Explosives
Safety Instruction for Hazardous Maerials (Appendix 3-4) Structures and Generation of Explosives "Safety Guidance for Chemical Experiments 4th Edition" ed. by The Chemical Society of Japan, Maruzen (1999) Bond Explosive compounds Chemical structures Generation reaction O-O High concentrated H2O2 hydrogen peroxide Organic peroxides R-O-O-R' Alcohols + H2O2 Halides + H2O2 Aldehydes + H2O2 Ketones + H2O2 Carboxylic acids + H2O2 Ethers + H2O2 O Ozonides Unsaturated hydrocarbon + O O O 3 Alcohols + HNO N-O Nitric acid esters C ONO2 3 Carbohydrates + HNO3 Nitrous acid esters C ONO Alcohols + HNO2 Halides + MNO2 Halides + M'NO2 Nitro compounds C NO2 Hydrocarbons, others + HNO3 Amine salts of nitric acid N HNO3 Amines + HNO3 Ammonium nitrate NH4NO3 NH3 + HNO3 Dehydration of amino nitrates Nitramines C N NO2 Amines + HNO3 Nitroso compounds C NO Phenols + HNO2 Ketone oximes O C C NOH R1 Hydroxyamine N OH derivatives R2 Fulminates M' O N C Metal nitrates + HNO3 + Alcohls X-O Dichlorine Heptoxide Cl2O7 Amine salts of perchloric acid N HClO4 Perchloric acid esters C OClO3 Alcohols + HClO4 Perchloryl compounds C ClO3 C H + FClO3 Chloric acid HClO3 M'ClO3 + Acids Chlorine dioxide ClO2 M'ClO2 + Acids Heavy metal salts of KClO + Heavy metal salts, M'ClO 3 chloric acid 3 Hg, Ag, Pb Ammonium chlorate NH ClO M'ClO3 + NH3 or 4 3 Ammonium salts Amine salts of chloric N HClO3 M'ClO + Amine salts acid 3 Chloric acid esters C OClO2 Chlorous acid HClO2 Hypochlorous acid HClO Chlorine monooxide Cl2O Salts of hypochlorous acid M'ClO Hypochlorous acid esters C OCl -

Chlorine Oxides and Chlorine Oxygen Acids 1
Chlorine Oxides and Chlorine Oxygen Acids 1 Chlorine Oxides and Chlorine Oxygen Acids Helmut Vogt, Technische Fachhochschule Berlin, Berlin, Federal Republic of Germany (Chaps. 1, 4.2 – 4.6, 7, and 9) Jan Balej, Ingenieurburo¨ fur¨ chemische Technik, Julich,¨ Federal Republic of Germany (Chap. 1) John E. Bennett, Eltech Systems Corp., Fairport Harbor, Ohio 44077, United States (Chaps. 4.2 – 4.6) Peter Wintzer, Cellulosefabrik Attisholz AG, Luterbach, Switzerland (Chaps. 7 and 9) Saeed Akbar Sheikh, Davy McKee AG, Frankfurt, Federal Republic of Germany (Chaps. 2, 3, 4.1, 5, 6, and 9) Patrizio Gallone, Politecnico di Milano, Milano, Italy (Chaps. 8 and 9) 1. Introduction ............... 2 7. Chloric Acid and Chlorates ..... 21 2. Hypochlorous Acid ........... 5 7.1. Properties ................. 22 3. Solid Hypochlorites ........... 5 7.2. Production Fundamentals ...... 24 3.1. Properties ................. 5 7.2.1. Chlorate-Generating Reactions .... 24 3.2. Production ................ 6 7.2.2. Loss Reactions .............. 26 3.3. Quality Specifications ......... 7 7.3. Industrial Electrosynthesis Systems 27 3.4. Uses ..................... 7 7.3.1. Electrolysis Cell Types ......... 28 4. Hypochlorite Solutions ........ 7 7.3.2. Electrodes ................. 28 4.1. Chemical Production .......... 7 7.3.3. Operational Parameters ......... 31 ............... 4.1.1. From Chlorine 8 7.3.4. Brine Purification ............. 33 ........ 4.1.2. From Bleaching Powder 8 7.4. Crystallization .............. 33 ............. 4.2. Electrosynthesis 9 7.5. Construction Materials ........ 33 ......... 4.2.1. Reaction Fundamentals 9 7.6. Environmental Protection ...... 34 .............. 4.2.2. Industrial Cells 10 7.7. Quality Specifications ......... 34 ................... 4.3. Storage 14 7.8. Storage, Transportation, and Safety 34 4.4. -

Chapter 5 Compounds and Their Bonds an Octet Refers to 8 Valence Electrons
Chapter 5 Compounds and Their Bonds An octet refers to 8 valence electrons This is associated with the stability of the noble gases other than He; He is stable with 2 valence electrons (duet) Valence Electrons He 1s2 2 Ne 1s2 2s2 2p6 8 Ar 1s2 2s2 2p6 3s2 3p6 8 One of the ways chemists have of explaining the formation of both inorganic and organic compounds and their relative stability is to view their reactivity as a attempt to become isoelectronic with either helium or one of the other inert gases ••• Li• + :Cl: = Li + :Cl: -1 ionic bond • ••• • • •• •• •• :Cl . + .Cl : = :Cl : Cl : covalent bond •• •• •• •• Sodium chloride, or “table salt,” is an example of an ionic compound An ionic formula consists of positively and negatively charged ions, is neutral, has charge balance total positive charge = total negative charge The symbol of the metal is written first, followed by the symbol of the nonmetal. In MgCl2, a Mg atom loses two valence electrons two Cl atoms each gain one electron subscripts indicate the number of ions needed to give charge balance In Na2S, two Na atoms lose one valence electron; each S atom gains two electrons; subscripts show the number of ions needed to give charge balance Select the correct formula for each of the following ionic compounds: A. Na+ and S2– 1) NaS 2) NaS2 3 Na2S B. Al3+ and Cl– 1) AlCl3 2) AlCl 3) A13Cl C. Mg2+ and N3– 1) MgN 2) Mg2N3 3) Mg3N2 Note that Mg+2 and Mg++ means the same as Mg2+ Formula Ions Name Cation Anion NaCl Na+ Cl– sodium chloride + 2– K2SK S potassium sulfide MgO Mg2+ O2– magnesium oxide 2+ – CaI2 Ca I calcium iodide 3+ 2– Al2S3 Al S aluminum sulfide Most transition metals form 2 or more positive ions Zn2+, Ag+, and Cd2+ form only one ion. -

Period: ___Covalent Bonding(Pg 583) Some Atoms of Non Metals
Name: __________________________________________ Period: ______ Covalent Bonding(pg 583) Some atoms of non metals are ____________________ to lose or gain electrons. For examples, The elements in Group 4 of the periodic table have _____________ electrons in their outer energy level. These atoms become chemically stable by _______________________ electrons rather than by losing or gaining electrons The attraction that forms between atoms when they share electrons is known as a ______________________ bond. A neutral particle that forms as the result of electron sharing is called a __________________________. Single Covalent Bonds A single covalent bond is made of __________________ shared electrons A water molecule contains two _____________________ bonds. The result of this type of bonding is a _______________________ outer energy level for each atom in the _______________________________. Multiple Bonds A covalent bond also can contain more than one ________________ of electrons A ______________ atom has five electrons in its outer energy level and needs ___________ electrons to become stable. It does this by _________________ three electrons with another Nitrogen atom. Covalent bonds form between _____________________ elements. Many covalent compounds are _____________________ or _____________________ at room temperature. **Polar or Non Polar** An unequal sharing of electrons give each chlorine atom a slight __________________ charge and each hydrogen atom a slightly ________________________ charge. The charge is ______________________ but not equally distributed, resulting in a __________________ molecule. A ______________________ molecule is one that has a slightly ___________________ end and a slightly ____________________end although the overall charge is neutral. Ammonia and Water are examples of Polar Molecules Two atoms that are exactly alike can share their electrons equally, forming a nonpolar molecule. -

Version 001 – Practice Exam 2 – Mccord – (49230) 1 This Print-Out
Version001–PracticeExam2–mccord–(49230) 1 b b This print-out should have 25 questions. b b b b 5. Ca + Ca + I b −→ Multiple-choice questions may continue on b − the next column or page – find all choices b b 2 + + b b before answering. Ca + Ca + b I b −→ Ca2I bb b b b b b b b b b b b b Important Message: Please please 6. Ca + b I b + b I b + b I b −→ b b b b b b − − − please remember to CORRECTLY bubble in b b b b b b 3+ b b b b b b your name, uteid, and version number for Ca + b I b + b I b + b I b −→ your exam. We are averaging about 20 stu- bb bb bb dents who fail to do this. Think of it as the Ca2I3 easiest question on the exam. If you get it b b b b wrong, you’ll get a zero when we grade it. Be- 7. Ca + b I b −→ b b − cause Quest is not that user friendly, it will b b + b b take at least 24 hours to get your avoidable Ca + b I b −→ CaI mistake fixed. It is a huge pain in the ass - bb b b know that. Please take the time to bubble b b b 8. Ca b + I b −→ in your information carefully. Lets have NO b − mistakes on this information please. Thank b b 2 2+ b b you so much. I’m only putting this plea on Ca + b I b −→ CaI bb this practice exam and not on the real exam. -

Modern Chemistry Name Chapter 7 Chemical Formulas and Chemical Compounds
Modern Chemistry Name Chapter 7 Chemical Formulas and Chemical Compounds Type I Binary Ionic Compounds Name the following compounds. Formula of compound Name of compound NaCl KBr Li2S AlCl3 RbF Ca2N3 Na2S LiCl SrO CsF MgI2 BeO KF Al2O3 NaF Write the formulas for the following compounds. Name of Compound Formula of compound Sodium oxide Lithium fluoride Magnesium chloride Calcium sulfide Potassium selenide Rubidium bromide Potassium iodide Strontium fluoride Cesium sulfide Barium bromide 4 Modern Chemistry Name Chapter 7 Chemical Formulas and Chemical Compounds Calcium phosphide Lithium nitride Barium sulfide Strontium iodide Rubidium chloride Type II Binary Ionic Compounds Write the formulas for the following compounds. Name of compound Formula of compound Iron III oxide Lead IV oxide Tin II sulfide Manganese II iodide Chromium III chloride Molybdenum IV bromide Nickel II chloride Copper I oxide Tungsten III sulfide Titanium III phosphide Lead IV fluoride Iridium II oxide Write the names of the following compounds (be sure to use Roman Numerals). Formula of compound Name of compound PbO CuF2 Ni2O3 Co3N2 CrS3 SnI4 PtO 5 Modern Chemistry Name Chapter 7 Chemical Formulas and Chemical Compounds CuO Nb2O5 Fe2O3 PdCl2 VP Compounds Containing Polyatomic Ions Name the following compounds containing polyatomic ions. Formula of compound Name of compound NaClO2 K2SO3 (NH4)2CrO4 Al2(SO4)3 Ca(NO2)2 Fe(OH)3 KMnO4 PbSO4 Co(NO2)2 Mn(NO3)2 NaClO Li2S2O3 Write the formulas for the following compounds. Name of compound Formula of compound Copper II chromate Cobalt II acetate Lead II azide Copper I carbonate Cobalt II phosphate Iron III bromate 6 Modern Chemistry Name Chapter 7 Chemical Formulas and Chemical Compounds Ammonium oxalate Potassium periodate Barium arsenate Zinc tartrate Silver chromate Aluminum citrate Type III Compounds (Molecules) Name the following molecules. -

Naming Compounds
Naming Compounds 3- PO4 phosphate ion HC2H3O2 Acetic Acid - C2H3O2 acetate ion Forms of Chemical Bonds There are 3 forms bonding atoms: Ionic complete transfer of 1 or more electrons from one atom to another (one loses, the other gains) Covalent some valence electrons shared Most bonds are between atoms somewhere in between ionic Metallic holds atoms of a metal together and covalent. Common Names • A lot of chemicals have common names as well as the proper IUPAC name. • Chemicals that should always be named by common name and never named by the IUPAC method are: • H2O water, not dihydrogen monoxide • NH3 ammonia, not nitrogen trihydride Compounds Formed From Ions CATION + ANION ---> COMPOUND Na+ + Cl- --> NaCl A neutral compound requires equal number of + and - charges. Predicting Charges on Monatomic Ions KNOW THESE !!!! +1 +2 +3 -3 -2 -1 0 Cd+2 Properties of Ionic Compounds Forming NaCl from Na and Cl2 • A metal atom can transfer an electron to a nonmetal. • The resulting cation and anion are attracted to each other by electrostatic forces. IONIC COMPOUNDS + NH4 Cl- ammonium chloride, NH4Cl Some Ionic Compounds 2+ - Ca + 2 F ---> CaF2 Mg2+ + N-3 ----> Mg3N2 magnesium nitride calcium fluoride Sn4+ + O2- ----> SnO2 Tin (IV) oxide Formulas of Ionic Compounds Formulas of ionic compounds are determined from the charges on the ions atoms ions – Na + F : Na+ : F : NaF sodium + fluorine sodium fluoride formula Charge balance: 1+ 1- = 0 Monatomic Ions Writing a Formula Write the formula for the ionic compound that will form between Ba2+ and Cl. Solution: 1. Balance charge with + and – ions 2. -

Unit 2 – Interim Review for Test 1 Electronic Configuration at the Atomic State Changes After Ionic Bonding: Follow the Model to Complete the Rest
Unit 2 – Interim Review for Test 1 Electronic Configuration at the atomic state changes after Ionic bonding: Follow the model to complete the rest Original Electronic Original Electronic electronic configuration electronic configuration Shell # of Element configuration After Bonding Element configuration After Bonding electrons H 1 2 K 2 Li 2, 1 2 F 2,7 2,8 L 8 Na 2,8,1 2,8 Cl 2,8,7 2,8,8 M 18 K 2,8, 9 2,8,8 Br 2,8,18,7 2,8,18,8 N 32 Group 1 Elements Group 17 Elements called Halogens O 32 P 18 In Ionic Bonding, atoms of elements gain or lose electrons to attain the noble gas Q 8 electronic configuration. In the first group of the periodic table, hydrogen is an exception – it is a nonmetal. The rest of them from Lithium to Francium are metals. What type of bond is formed They have one electron in excess of the nearest noble gas, which precedes them in the between alkali metals and periodic table. Therefore, these metals will lose one electron to attain the noble gas halogens? Remember hydrogen is configuration. a nonmetal and do not include in th th Halogens are nonmetals and they are Fluorine through Tennessine, on the 7 (or 17 ) alkali metals. group of the periodic table. They have one electron less than the nearest noble gas, Answer: Ionic Bond which follows them on the periodic table. Therefore, halogens will gain one electron to attain the noble gas configuration. Important Note: Covalent compounds are also known as Writing formula of Compounds Important Note: Using electronegativity, we can find Molecular compounds. -

Chemistry Fundamentals of Engineering Exam Review Other Disciplines FE Specifications Chemistry: 7–11 FE Exam Problems
Fundamentals of Engineering Exam Review Fundamentals of Engineering Exam Review Chemistry Fundamentals of Engineering Exam Review Other Disciplines FE Specifications Chemistry: 7–11 FE exam problems A. Periodic Table (e.g., nomenclature, metals and non-metals, atomic structure of matter)- 8Q’s B. Oxidation and Reduction – 4 Q’s C. Acids and Bases – 5 Q’s D. Equations (e.g., stoichiometry, equilibrium) – 7 Q’s E. Gas Laws (e.g., Boyle’s and Charles’ Laws, molar volume) – 4 Q’s Fundamentals of Engineering Exam Review A. PERIODIC TABLE: NOMENCLATURE, METALS AND NON-METALS The Periodic Table is divided into three regions: metals, non-metals, and metalloids Materials composed of all the same type of metal or non-metal are simply named by their element symbol. Proper nomenclature requires identifying if a compound is composed of: metals & non-metals (ionic) non-metals & non-metals (molecular) A. Periodic Table 3 Fundamentals of Engineering Exam Review metals metalloids non-metals (Periodic Table from chemistry section of FE Reference Handbook) A. Periodic Table 4 Fundamentals of Engineering Exam Review Ionic compounds are composed of metals and non-metals If the metal is from periodic table column I, column II, or aluminium, name of the compound with the metal name and the non-metal name with a substituted -IDE ending CaCl2 is calcium chlorIDE (chlorine chloride) Na2O is sodium oxIDE (oxygen oxide) Al2S3 is aluminium sulfIDE (sulfur sulfide) A. Periodic Table 5 Fundamentals of Engineering Exam Review Ionic compounds are composed of metals and non-metals If the metal is NOT FROM periodic table column 1, column 2, or aluminium, name of the compound with the metal name (roman mumber representing charge) and the non-metal name with a substituted -IDE ending FeCl2 is iron (II) chloride (each Cl is -1 so Fe is +2) TiO2 is titanium (IV) oxide (each O is -2 so Ti is +4) Tl2S3 is thallium (III) sulfide (each S is -2 so Tl is +3) A.