PLURISTEM THERAPEUTICS INC. (Exact Name of Registrant As Specified in Its Charter)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Advancing Cell Therapeutic Products for Clinical Use
Advancing cell therapeutic products for clinical use 1 Proprietary data of Pluristem Therapeutics Inc. Forward Looking Statement This presentation concerning Pluristem Therapeutics may include forward- looking statements which represent Pluristem Therapeutics' expectations or beliefs regarding future events. I caution that such statements involve risks and uncertainties that may cause actual results to differ materially from those in the forward-looking statements. Consequently, all such forward-looking statements are qualified by the cautionary language and risk factors set forth in Pluristem Therapeutics' periodic and other reports filed with the SEC. There can be no assurance that the actual results, events or developments referenced in such forward-looking statements will occur or be realized. Pluristem Therapeutics assumes no obligation to update these forward- looking statements to reflect actual results, changes in assumptions or changes in factors affecting such forward-looking statements. www.pluristem.com 2 Proprietary data of Pluristem Therapeutics Inc. Corporate Overview • Cell therapy company (NasdaqCM: PSTI, TASE: PSTI) • Using off-the-shelf, placenta-derived cells to achieve both local and systemic therapeutic effects – no tissue matching needed • First-in-class 3D cell culturing technology allowing for efficient, controlled production of multiple cell products in commercial quantities – “the process is the product” • Active with regulators in the U.S., EU, S. Korea, Australia & Israel • Demonstrated safety and efficacy in 3 clinical studies (two Phase I and one Phase I/II study) 3 Proprietary data of Pluristem Therapeutics Inc. Financial Overview • Market Cap: ~ $200 million • Cash and marketable securities: $41 million (March, 2015) • No debt • Net burn: ~ $23 million • 165 employees (16 PhD, 4 MD) • IP Ownership: over 40 granted patents and ~150 pending applications 4 Proprietary data of Pluristem Therapeutics Inc. -

Biolinerx Ltd. (Translation of Registrant’S Name Into English) ______
SECURITIES AND EXCHANGE COMMISSION WASHINGTON, D.C. 20549 FORM 6-K REPORT OF FOREIGN PRIVATE ISSUER PURSUANT TO RULE 13a-16 OR 15d-16 OF THE SECURITIES EXCHANGE ACT OF 1934 For the month of February 2018 _______________________ BioLineRx Ltd. (Translation of registrant’s name into English) _______________________ 2 HaMa’ayan Street Modi’in 7177871, Israel (Address of Principal Executive Offices) _______________________ Indicate by check mark whether the registrant files or will file annual reports under cover of Form 20-F or Form 40-F: Form 20-F ☑ Form 40-F ☐ Indicate by check mark whether the registrant by furnishing the information contained in this form is also thereby furnishing the information to the Commission pursuant to Rule 12g3-2(b) under the Securities Exchange Act of 1934: Yes ☐ No ☑ The registrant hereby announces that it has entered into an employment agreement with Hillit Mannor Shachar, M.D., MBA, M.S.F.S., to serve as its new Vice President Business Development, effective April 1, 2018. Dr. Shachar will be responsible for BioLineRx’s business development, commercialization of assets and pipeline strategy. Dr. Shachar joins BioLineRx with over 15 years of experience in senior business development, corporate development and venture capital positions in the life sciences field. Her recent experience has included service as Vice President Business Development of Pluristem Therapeutics (NASDAQ:PSTI), a leading developer of placenta-based cell therapy products, and Director of New Business Development at West Pharmacuetical Services. In addition, Dr. Shachar has served at several life science companies and venture capital funds, including Apax Partners, Nektar Therapeutics, Orex Computed Radiography, Kodak and Transpharma Medical. -

Life Sciences in Israel
STATE OF ISRAEL Ministry of Industry Trade and Labor Investment Promotion Center Inspiration Invention Innovation Life Sciences in Israel www.investinisrael.gov.il Table of Contents .......................................... 3 Israel: A Powerhouse of Opportunities ............................................................ 3 Israel’s Life Science Sectors Medical Devices ........................................................................ 4 Healthcare IT ............................................................................ 4 BioPharmaceutical .................................................................... 7 Israel’s Biomedical Engineering - ................................................... 11 Spotlight on Stem Cell Research ....................................... 15 Israel’s Life Sciences Competitive Edge ..................................................................... 19 Government Support 2 Life Sciences in Israel Israel: A Powerhouse of Opportunities Why Israel’s Life Sciences Over the last decade, Israel has introduced a wealth of groundbreaking More than 1,000 Life and valuable innovations in Life Sciences. Israel’s Life Sciences sector Sciences Companies - is supported by a strong foundation of academic excellence, including Biopharma and Medical some of the world’s leading research institutes, renowned R&D Devices facilities and cutting-edge medical centers. Bolstered by a highly skilled Over 1/3 of LS Start-Ups workforce, a flourishing high-tech environment, and an entrepreneurial already generate revenue -

Fidelity® Nasdaq Composite Index® Fund
Fidelity® Nasdaq Composite Index® Fund Semi-Annual Report May 31, 2021 Contents Note to Shareholders 3 Investment Summary 4 Schedule of Investments 6 Financial Statements 85 Notes to Financial 89 Statements Shareholder Expense 97 Example Board Approval of 98 Investment Advisory Contracts and Management Fees Liquidity Risk 106 Management Program To view a fund’s proxy voting guidelines and proxy voting record for the 12-month period ended June 30, visit http://www.fidelity.com/proxyvotingresults or visit the Securities and Exchange Commission’s (SEC) web site at http://www.sec.gov. You may also call 1-800-544-8544 to request a free copy of the proxy voting guidelines. Nasdaq®, OMX®, NASDAQ OMX®, Nasdaq Composite®, and The Nasdaq Stock Market®, Inc. are registered trademarks of The NASDAQ OMXGroup, Inc. (which with its Affiliates are the Corporations) and are licensed for use by Fidelity. The product has not been passed on by the Corporations as to its legality or suitability. The product is not issued, endorsed or sold by the Corporations. The Corporations make no warranties and bear no liability with respect to shares of the product. Standard & Poor’s, S&P and S&P 500 are registered service marks of The McGraw-Hill Companies, Inc. and have been licensed for use by Fidelity Distributors Corporation. Other third-party marks appearing herein are the property of their respective owners. All other marks appearing herein are registered or unregistered trademarks or service marks of FMR LLC or an affiliated company. © 2021 FMR LLC. All rights reserved. This report and the financial statements contained herein are submitted for the general information of the shareholders of the Fund. -

Fidelity® Nasdaq Composite Index® Fund
Quarterly Holdings Report for Fidelity® Nasdaq Composite Index® Fund February 28, 2021 EIF-QTLY-0421 1.814098.116 Schedule of Investments February 28, 2021 (Unaudited) Showing Percentage of Net Assets Common Stocks – 99.7% Shares Value COMMUNICATION SERVICES – 16.7% Diversified Telecommunication Services – 0.2% Alaska Communication Systems Group, Inc. 34,501 $ 112,818 Anterix, Inc. (a) 7,844 331,252 ATN International, Inc. 7,220 351,470 Bandwidth, Inc. (a) (b) 12,082 1,913,306 Cogent Communications Group, Inc. (b) 25,499 1,526,115 Consolidated Communications Holdings, Inc. (a) 21,768 114,500 Iridium Communications, Inc. (a) 77,117 2,954,352 Liberty Global PLC: Class A (a) 112,326 2,766,028 Class B (a) 327 7,521 Class C (a) 204,417 4,967,333 Liberty Latin America Ltd.: Class A (a) 17,405 190,933 Class C (a) 105,781 1,159,360 ORBCOMM, Inc. (a) 54,925 419,078 Radius Global Infrastructure, Inc. (a) (b) 37,222 460,808 Sify Technologies Ltd. sponsored ADR (a) (b) 7,275 22,916 Vonage Holdings Corp. (a) 142,421 1,882,806 19,180,596 Entertainment – 2.5% Activision Blizzard, Inc. 429,734 41,086,868 Bilibili, Inc. ADR (a) (b) 99,200 12,496,224 Blue Hat Interactive Entertainment Technology (a) (b) 13,117 16,659 Chicken Soup For The Soul Entertainment, Inc. (a) 2,009 51,370 Cinedigm Corp. (a) 73,305 102,627 CuriosityStream, Inc. Class A (a) 24,573 426,833 DouYu International Holdings Ltd. ADR (a) 82,330 1,180,612 Electronic Arts, Inc. -

Nasdaq Capital Market Friday, December 28, 2018 5:32 PM
Nasdaq Capital Market Friday, December 28, 2018 5:32 PM Name Symbol Close 3PEA International TPNL 3.35 Abeona Therapeutics ABEO 7.02 Ability ABIL 2.02 Abraxas Petroleum AXAS 1.08 Acasti Pharma ACST 0.73 Accelerate Diagnostics AXDX 11.78 Acer Therapeutics ACER 18.25 Achieve Life Sciences ACHV 1.2 ACNB ACNB 38.73 Adamis Pharmaceuticals ADMP 2.1 Adesto Technologies IOTS 4.3 ADial Pharmaceuticals ADIL 5.55 ADMA Biologics ADMA 2.23 ADOMANI ADOM 0.25 Aehr Test Systems AEHR 1.44 Aerpio Pharmaceuticals ARPO 1.71 AEterna Zentaris AEZS 2.97 Aethlon Medical AEMD 1.52 Agenus AGEN 2.39 AGM Group Holdings AGMH 28.05 Airgain AIRG 10.04 AirMedia Group ADR AMCN 0.24 Air T AIRT 24.03 Akari Therapeutics ADR AKTX 1.63 Akers Biosciences AKER 1.27 Akoustis Technologies AKTS 4.79 Alberton Acquisition Cl A ALAC 9.8 Alberton Acquisition WT ALACW 0.12 Albireo Pharma ALBO 23.18 Aldeyra Therapeutics ALDX 8 Alithya Group Cl A ALYA 2.55 Alkaline Water WTER 3.24 Allegro Merger Rt ALGRR 0.3 Alliance MMA AMMA 0.17 Allied Healthcare Products AHPI 1.89 Alliqua BioMedical ALQA 2 Alta Mesa Resources AMR 1 Alta Mesa Resources WT AMRWW 0.08 Altus Midstream ALTM 7.75 AMCI Acquisition AMCIU 9.95 American Electric Technologies AETI 0.89 Ameri Holdings AMRH 0.17 Ameri Holdings WT AMRHW 0.03 Ames National ATLO 25.72 Anavex Life Sciences AVXL 1.52 Anixa Biosciences ANIX 4 Antares Pharma ATRS 2.63 Apollo Medical Holdings AMEH 20.73 Appliance Recycling Centers of ARCI 0.5 America Applied DNA Sciences APDN 0.38 Applied DNA Sciences WT APDNW .. -

LD Micro Index Feb 2021
Symbol Name Stock Exchange FactSet Sector Market Capitalization SRAX SRAX INC NASDAQ Technology $49,843,005.90 BCDA BioCardia, Inc. NASDAQ Healthcare $50,057,999.80 LOAN Manhattan Bridge Capital, Inc. NASDAQ Finance $50,119,913.45 CCLP CSI Compressco LP NASDAQ Industrial Services $50,193,428.46 Ambow Education Holding Ltd. Sponsored AMBO ADR Class A NYSE AMERICAN Consumer $50,377,281.29 SSKN STRATA Skin Sciences, Inc. NASDAQ Healthcare $50,654,863.50 N Namaste Technologies, Inc. TSX VENTURE Consumer $50,848,294.31 DFFN Diffusion Pharmaceuticals Inc. NASDAQ Healthcare $50,892,275.60 BPT BP Prudhoe Bay Royalty Trust NYSE Miscellaneous $50,932,000.00 EKSO Ekso Bionics Holdings, Inc. NASDAQ Healthcare $50,978,968.04 NBRV Nabriva Therapeutics Plc NASDAQ Healthcare $51,010,650.02 DMGI DMG Blockchain Solutions, Inc. TSX VENTURE Finance $51,022,176.75 PW Power REIT NYSE AMERICAN Finance $51,180,072.69 INPX Inpixon NASDAQ Technology $51,182,031.24 HFEN HF Enterprises Inc NASDAQ Finance $51,248,600.00 LLG Mason Graphite, Inc. TSX VENTURE Minerals $51,317,878.52 GOED 1847 Goedeker Inc NYSE AMERICAN Consumer $51,456,304.00 PHX PHX Minerals Inc. Class A NYSE Minerals $51,495,146.20 MNDO MIND C.T.I. Ltd. NASDAQ Commercial Services $51,531,484.34 ANVS Annovis Bio Inc NYSE AMERICAN Healthcare $51,962,724.32 PIXY ShiftPixy, Inc. NASDAQ Commercial Services $52,046,343.54 SGBX SG Blocks, Inc. NASDAQ Producer Manufacturing$52,436,752.90 QST Questor Technology Inc. TSX VENTURE Minerals $52,488,379.23 SEAC SeaChange International, Inc. -

ARK Invest Change to the Founds Holdings
IZRL - Israel Innovative Technology ETF page 1 of 8 Assets that ARK invest Sold out off No new assets detected page 2 of 8 New assets that ARK invest have added to their funds No new assets detected page 3 of 8 Assets where ARK invest decrease number of shares No new assets detected page 4 of 8 Assets where ARK invest increase number of shares Compared to 04-12-2020 Date Fund Ticker Shares bought Shares bought % Company 07-12-2020 IZRL AYLA 3.750 5,28% AYALA PHARMACEUTICALS INC 07-12-2020 IZRL SSYS 3.345 4,92% STRATASYS LTD 07-12-2020 IZRL CGEN 3.039 4,67% COMPUGEN LTD 07-12-2020 IZRL PSTI 4.752 4,47% PLURISTEM THERAPEUTICS INC 07-12-2020 IZRL RADA 7.410 4,45% RADA ELECTRONIC INDS LTD 07-12-2020 IZRL FTAL 744 4,45% FATTAL HOLDINGS 1998 LTD 07-12-2020 IZRL PERI 6.441 4,44% PERION NETWORK LTD 07-12-2020 IZRL NVMI 849 4,40% NOVA MEASURING INSTRUMENTS 07-12-2020 IZRL DANE 399 4,39% DANEL (ADIR YEOSHUA) LTD 07-12-2020 IZRL BVXV 1.218 4,39% BIONDVAX PHARMACEUTICALS-ADR 07-12-2020 IZRL INMD 1.209 4,38% INMODE LTD 07-12-2020 IZRL WIX 171 4,37% WIX.COM LTD 07-12-2020 IZRL TARO 819 4,37% TARO PHARMACEUTICAL INDUS 07-12-2020 IZRL RDHL 4.872 4,37% REDHILL BIOPHARMA LTD-SP ADR 07-12-2020 IZRL MTRX 2.025 4,36% MATRIX IT LTD 07-12-2020 IZRL CRNT 19.050 4,36% CERAGON NETWORKS LTD 07-12-2020 IZRL CYBR 435 4,36% CYBERARK SOFTWARE LTD/ISRAEL 07-12-2020 IZRL CAMT 2.664 4,35% CAMTEK LTD 07-12-2020 IZRL TEVA 5.238 4,35% TEVA PHARMACEUTICAL-SP ADR 07-12-2020 IZRL HLAN 1.116 4,35% HILAN LTD 07-12-2020 IZRL AUDC 1.458 4,34% AUDIOCODES LTD 07-12-2020 IZRL ESLT -
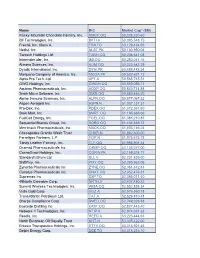
Downloadable PDF of Index Constituents
Name RIC Market Cap* ($M) Rocky Mountain Chocolate Factory, Inc. RMCF.OQ 50,028,230.60 BK Technologies, Inc. BKTI.A 50,095,548.75 Freshii, Inc. Class A FRII.TO 50,129,434.95 Netlist, Inc. NLST.PK 50,130,950.04 Tantech Holdings Ltd. TANH.OQ 50,204,641.08 Intermolecular, Inc. IMI.OQ 50,250,041.16 Alimera Sciences, Inc. ALIM.OQ 50,323,642.29 Dyadic International, Inc. DYAI.PK 50,488,488.54 Marijuana Company of America, Inc. MCOA.PK 50,532,621.12 Alpha Pro Tech, Ltd. APT.A 50,565,745.51 GWG Holdings, Inc. GWGH.OQ 50,569,086.11 Aquinox Pharmaceuticals, Inc. AQXP.OQ 50,840,714.88 Smith Micro Software, Inc. SMSI.OQ 50,880,445.20 Alpine Immune Sciences, Inc. ALPN.OQ 50,977,067.52 Aspen Aerogels Inc ASPN.N 51,057,137.31 Pro-Dex, Inc. PDEX.OQ 51,072,367.60 Stein Mart, Inc. SMRT.OQ 51,195,688.66 FuelCell Energy, Inc. FCEL.OQ 51,385,210.85 Sequential Brands Group, Inc. SQBG.OQ 51,434,659.73 Merrimack Pharmaceuticals, Inc. MACK.OQ 51,503,146.24 Chesapeake Granite Wash Trust CHKR.N 51,892,500.00 Ferrellgas Partners, L.P. FGP.N 51,976,675.78 Tandy Leather Factory, Inc. TLF.OQ 51,995,901.44 Oramed Pharmaceuticals Inc. ORMP.OQ 52,135,077.00 CannaGrow Holdings, Inc. CGRW.PK 52,159,524.77 Standard Lithium Ltd. SLL.V 52,231,839.00 ShiftPixy, Inc. PIXY.OQ 52,280,962.06 Zynerba Pharmaceuticals Inc ZYNE.OQ 52,351,812.81 Conatus Pharmaceuticals Inc. -
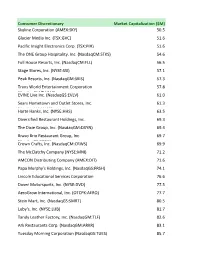
Index Constitutents To
Consumer Discretionary Market Capitalization ($M) Skyline Corporation (AMEX:SKY) 50.5 Glacier Media Inc. (TSX:GVC) 51.6 Pacific Insight Electronics Corp. (TSX:PIH) 51.6 The ONE Group Hospitality, Inc. (NasdaqCM:STKS) 54.6 Full House Resorts, Inc. (NasdaqCM:FLL) 56.5 Stage Stores, Inc. (NYSE:SSI) 57.1 Peak Resorts, Inc. (NasdaqGM:SKIS) 57.3 Trans World Entertainment Corporation 57.8 EVINE Live Inc. (NasdaqGS:EVLV)(NasdaqGM:TWMC) 61.0 Sears Hometown and Outlet Stores, Inc. 61.3 Harte Hanks, Inc. (NYSE:HHS)(NasdaqCM:SHOS) 63.5 Diversified Restaurant Holdings, Inc. 69.3 The Dixie Group, Inc. (NasdaqGM:DXYN)(NasdaqCM:SAUC) 69.4 Bravo Brio Restaurant Group, Inc. 69.7 Crown Crafts, Inc. (NasdaqCM:CRWS)(NasdaqGS:BBRG) 69.9 The McClatchy Company (NYSE:MNI) 71.2 AMCON Distributing Company (AMEX:DIT) 71.6 Papa Murphy's Holdings, Inc. (NasdaqGS:FRSH) 74.1 Lincoln Educational Services Corporation 76.6 Dover Motorsports, Inc. (NYSE:DVD)(NasdaqGS:LINC) 77.5 AeroGrow International, Inc. (OTCPK:AERO) 77.7 Stein Mart, Inc. (NasdaqGS:SMRT) 80.5 Luby's, Inc. (NYSE:LUB) 81.7 Tandy Leather Factory, Inc. (NasdaqGM:TLF) 82.6 Ark Restaurants Corp. (NasdaqGM:ARKR) 83.1 Tuesday Morning Corporation (NasdaqGS:TUES) 85.7 UCP, Inc. (NYSE:UCP) 87.1 Universal Technical Institute, Inc. (NYSE:UTI) 88.3 New York & Company, Inc. (NYSE:NWY) 88.6 Cherokee Inc. (NasdaqGS:CHKE) 90.0 JAKKS Pacific, Inc. (NasdaqGS:JAKK) 91.1 Torstar Corporation (TSX:TS.B) 92.6 Unique Fabricating, Inc. (AMEX:UFAB) 92.9 Ballantyne Strong, Inc (AMEX:BTN) 95.5 Bluestem Group Inc. -

Index Announcement
INDEX ANNOUNCEMENT S&P Dow Jones Indices announces changes to the S&P / Harel Sector Indices London, March 25, 2014 – The S&P / Harel Indices are being rebalanced after the close of trading on Monday, March 31st. To follow are the list of indices, their constituents and weights, effective on that date. S&P / Harel Consumer Goods Index Name Weight Osem Investment 15.000% Strauss Group 15.000% Rami Levi Chain Stores Hashikma Marketing Ltd. 15.000% Delek Automotive Systems Ltd 13.123% FOX WIZEL LTD 12.116% Shufersal Ltd. 9.723% Delta-Galil Industries 8.012% Kerur Hldgs 1 3.139% CARASSO MOTORS LTD 2.133% Maabarot Products Ltd. 1.382% Neto ME Holdings Ltd 1.372% Alon Blue Square Israel Ltd 1.000% Gan Shmuel Food Industries 1.000% Dor Alon Energy In Israel (1988) 1.000% Neto Malinda Trading Ltd. 1.000% McGRAW-HILL S&P DOW JONES INDICES INDEX ANNOUNCEMENT S&P / Harel Energy Index Name Weight Isramco Negev 2 LP 15.000% Delek Group Ltd 15.000% Ratio Oil Exploration L.P. 15.000% Avner Oil & Gas Ltd LP 14.776% Paz Oil Company Ltd 11.899% Delek Drilling LP 10.483% Oil Refineries Ltd 5.959% Ormat Industries 4.638% Delek Energy Systems Ltd 1.245% Naphtha Israel Petroleum Corp 1.000% I.N.O.C.-Dead Sea L.P. 1.000% Givot Olam Oil Exploration L.P. 1.000% Alon Natural Gas Exploration Ltd. 1.000% Naphtha Explorations L.P. 1.000% LAPIDOTH-HELETZ LP 1.000% S&P / Harel Health Care Index Name Weight Teva Pharmaceutical Industries 15.000% Perrigo Company plc 15.000% Mazor Robotics Ltd. -

Israel's Life Sciences Industry IATI Report 2017
With the support of Israel’s Life Sciences Industry IATI Report 2017 Connecting Israel’s Tech Ecosystem ISRAEL'S UMBRELLA ORGANIZATION for the High-Tech and Life Science Industries Connecting the tech ecosystem IATI.CO.IL To learn more about joining IATI: T: +972 73713 6313 / [email protected] / www.iati.co.il Herzliya Pituach, Israel Dear Friends, The IATI 2017 Report: Israel’s Life Sciences Industry offers a sweeping panorama of the country’s flourishing life sciences industry, which is growing in prominence in the global healthcare market. The report provides a snapshot of various aspects of the industry, from current trends and influences to systematic elements including continued government support and academic excellence. The Israeli Life Sciences Industry has accelerated rapidly, with a growing pipeline in a variety of industry segments. This growth is aided by consistent government support and a strong academic base, which is a leading source for companies and technologies within the ecosystem. We at IATI, Israel’s Umbrella Organization of the High-Tech & Life Science industries, believe that the combination of the country’s experienced and highly-educated professionals, an outstanding academic community, innovative spirit and technological prowess will only propel Israel’s life sciences industry even further in the coming years. We expect more cooperation between multinational and local players to bring revolutionary treatments and solutions to people around the world. This unique report provides an in-depth view of the local industry, highlighting the sustained growth and progress that was made and help give readers an idea of where the industry is headed.