Patterns and Processes of Shell Fragmentation in Modern and Ancient Marine Environments
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Impacts of Climate Change on Marine Fisheries and Aquaculture in Chile
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/319999645 Impacts of Climate Change on Marine Fisheries and Aquaculture in Chile Chapter · September 2017 DOI: 10.1002/9781119154051.ch10 CITATIONS READS 0 332 28 authors, including: Nelson A Lagos Ricardo Norambuena University Santo Tomás (Chile) University of Concepción 65 PUBLICATIONS 1,052 CITATIONS 13 PUBLICATIONS 252 CITATIONS SEE PROFILE SEE PROFILE Claudio Silva Marco A Lardies Pontificia Universidad Católica de Valparaíso Universidad Adolfo Ibáñez 54 PUBLICATIONS 432 CITATIONS 70 PUBLICATIONS 1,581 CITATIONS SEE PROFILE SEE PROFILE Some of the authors of this publication are also working on these related projects: Irish moss - green crab interactions View project Influence of environment on fish stock assessment View project All content following this page was uploaded by Pedro A. Quijón on 11 November 2017. The user has requested enhancement of the downloaded file. 239 10 Impacts of Climate Change on Marine Fisheries and Aquaculture in Chile Eleuterio Yáñez1, Nelson A. Lagos2,13, Ricardo Norambuena3, Claudio Silva1, Jaime Letelier4, Karl-Peter Muck5, Gustavo San Martin6, Samanta Benítez2,13, Bernardo R. Broitman7,13, Heraldo Contreras8, Cristian Duarte9,13, Stefan Gelcich10,13, Fabio A. Labra2, Marco A. Lardies11,13, Patricio H. Manríquez7, Pedro A. Quijón12, Laura Ramajo2,11, Exequiel González1, Renato Molina14, Allan Gómez1, Luis Soto15, Aldo Montecino16, María Ángela Barbieri17, Francisco Plaza18, Felipe Sánchez18, -

EAA Meeting 2016 Vilnius
www.eaavilnius2016.lt PROGRAMME www.eaavilnius2016.lt PROGRAMME Organisers CONTENTS President Words .................................................................................... 5 Welcome Message ................................................................................ 9 Symbol of the Annual Meeting .............................................................. 13 Commitees of EAA Vilnius 2016 ............................................................ 14 Sponsors and Partners European Association of Archaeologists................................................ 15 GENERAL PROGRAMME Opening Ceremony and Welcome Reception ................................. 27 General Programme for the EAA Vilnius 2016 Meeting.................... 30 Annual Membership Business Meeting Agenda ............................. 33 Opening Ceremony of the Archaelogical Exhibition ....................... 35 Special Offers ............................................................................... 36 Excursions Programme ................................................................. 43 Visiting Vilnius ............................................................................... 57 Venue Maps .................................................................................. 64 Exhibition ...................................................................................... 80 Exhibitors ...................................................................................... 82 Poster Presentations and Programme ........................................... -

The Paleoecology and Biogeography of Ordovician Edrioasteroids
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Doctoral Dissertations Graduate School 8-2011 The Paleoecology and Biogeography of Ordovician Edrioasteroids Rene Anne Lewis University of Tennessee - Knoxville, [email protected] Follow this and additional works at: https://trace.tennessee.edu/utk_graddiss Part of the Paleontology Commons Recommended Citation Lewis, Rene Anne, "The Paleoecology and Biogeography of Ordovician Edrioasteroids. " PhD diss., University of Tennessee, 2011. https://trace.tennessee.edu/utk_graddiss/1094 This Dissertation is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a dissertation written by Rene Anne Lewis entitled "The Paleoecology and Biogeography of Ordovician Edrioasteroids." I have examined the final electronic copy of this dissertation for form and content and recommend that it be accepted in partial fulfillment of the requirements for the degree of Doctor of Philosophy, with a major in Geology. Michael L. McKinney, Major Professor We have read this dissertation and recommend its acceptance: Colin D. Sumrall, Linda C. Kah, Arthur C. Echternacht Accepted for the Council: Carolyn R. Hodges Vice Provost and Dean of the Graduate School (Original signatures are on file with official studentecor r ds.) THE PALEOECOLOGY AND BIOGEOGRAPHY OF ORDOVICIAN EDRIOASTEROIDS A Dissertation Presented for the Doctor of Philosophy Degree The University of Tennessee, Knoxville René Anne Lewis August 2011 Copyright © 2011 by René Anne Lewis All rights reserved. -

Download Book (PDF)
M o Manual on IDENTIFICATION OF SCHEDULE MOLLUSCS From India RAMAKRISHN~~ AND A. DEY Zoological Survey of India, M-Block, New Alipore, Kolkota 700 053 Edited by the Director, Zoological Survey of India, Kolkata ZOOLOGICAL SURVEY OF INDIA KOLKATA CITATION Ramakrishna and Dey, A. 2003. Manual on the Identification of Schedule Molluscs from India: 1-40. (Published : Director, Zool. Surv. India, Kolkata) Published: February, 2003 ISBN: 81-85874-97-2 © Government of India, 2003 ALL RIGHTS RESERVED • No part of this publication may be reproduced, stored in a retrieval system or transmitted, in any from or by any means, electronic, mechanical, photocopying, recording or otherwise without the prior permission of the publisher. • -This book is sold subject to the condition that it shall not, by way of trade, be lent, resold hired out or otherwise disposed of without the publisher's consent, in any form of binding or cover other than that in which it is published. • The correct price of this publication is the price printed on this page. Any revised price indicated by a rubber stamp or by a sticker or by any other means is incorrect and should be unacceptable. PRICE India : Rs. 250.00 Foreign : $ (U.S.) 15, £ 10 Published at the Publication Division by the Director, Zoological Survey of India, 234/4, AJ.C. Bose Road, 2nd MSO Building (13th Floor), Nizam Palace, Kolkata -700020 and printed at Shiva Offset, Dehra Dun. Manual on IDENTIFICATION OF SCHEDULE MOLLUSCS From India 2003 1-40 CONTENTS INTRODUcrION .............................................................................................................................. 1 DEFINITION ............................................................................................................................ 2 DIVERSITY ................................................................................................................................ 2 HA.B I,.-s .. .. .. 3 VAWE ............................................................................................................................................ -

71St Annual Meeting Society of Vertebrate Paleontology Paris Las Vegas Las Vegas, Nevada, USA November 2 – 5, 2011 SESSION CONCURRENT SESSION CONCURRENT
ISSN 1937-2809 online Journal of Supplement to the November 2011 Vertebrate Paleontology Vertebrate Society of Vertebrate Paleontology Society of Vertebrate 71st Annual Meeting Paleontology Society of Vertebrate Las Vegas Paris Nevada, USA Las Vegas, November 2 – 5, 2011 Program and Abstracts Society of Vertebrate Paleontology 71st Annual Meeting Program and Abstracts COMMITTEE MEETING ROOM POSTER SESSION/ CONCURRENT CONCURRENT SESSION EXHIBITS SESSION COMMITTEE MEETING ROOMS AUCTION EVENT REGISTRATION, CONCURRENT MERCHANDISE SESSION LOUNGE, EDUCATION & OUTREACH SPEAKER READY COMMITTEE MEETING POSTER SESSION ROOM ROOM SOCIETY OF VERTEBRATE PALEONTOLOGY ABSTRACTS OF PAPERS SEVENTY-FIRST ANNUAL MEETING PARIS LAS VEGAS HOTEL LAS VEGAS, NV, USA NOVEMBER 2–5, 2011 HOST COMMITTEE Stephen Rowland, Co-Chair; Aubrey Bonde, Co-Chair; Joshua Bonde; David Elliott; Lee Hall; Jerry Harris; Andrew Milner; Eric Roberts EXECUTIVE COMMITTEE Philip Currie, President; Blaire Van Valkenburgh, Past President; Catherine Forster, Vice President; Christopher Bell, Secretary; Ted Vlamis, Treasurer; Julia Clarke, Member at Large; Kristina Curry Rogers, Member at Large; Lars Werdelin, Member at Large SYMPOSIUM CONVENORS Roger B.J. Benson, Richard J. Butler, Nadia B. Fröbisch, Hans C.E. Larsson, Mark A. Loewen, Philip D. Mannion, Jim I. Mead, Eric M. Roberts, Scott D. Sampson, Eric D. Scott, Kathleen Springer PROGRAM COMMITTEE Jonathan Bloch, Co-Chair; Anjali Goswami, Co-Chair; Jason Anderson; Paul Barrett; Brian Beatty; Kerin Claeson; Kristina Curry Rogers; Ted Daeschler; David Evans; David Fox; Nadia B. Fröbisch; Christian Kammerer; Johannes Müller; Emily Rayfield; William Sanders; Bruce Shockey; Mary Silcox; Michelle Stocker; Rebecca Terry November 2011—PROGRAM AND ABSTRACTS 1 Members and Friends of the Society of Vertebrate Paleontology, The Host Committee cordially welcomes you to the 71st Annual Meeting of the Society of Vertebrate Paleontology in Las Vegas. -

Download Date 30/12/2018 22:47:41
Stratigraphy and paleontology of the Naco Formation in the southern Dripping Spring Mountains, near Winkelman, Gila County, Arizona Item Type text; Thesis-Reproduction (electronic); maps Authors Reid, Alastair Milne, 1940- Publisher The University of Arizona. Rights Copyright © is held by the author. Digital access to this material is made possible by the University Libraries, University of Arizona. Further transmission, reproduction or presentation (such as public display or performance) of protected items is prohibited except with permission of the author. Download date 30/12/2018 22:47:41 Link to Item http://hdl.handle.net/10150/551821 STRATIGRAPHY AND PALEONTOLOGY OF THE NACO FORMATION IN THE SOUTHERN DRIPPING SPRING MOUNTAINS, NEARWINKELMAN, GILA COUNTY, ARIZONA by Ala stair M. Reid A Thesis Submitted to the Faculty of the DEPARTMENT OF GEOLOGY In Partial Fulfillment of the Requirements For the Degree of MASTER OF SCIENCE In the Graduate College THE UNIVERSITY OF ARIZONA 1966 STATEMENT BY AUTHOR This thesis has been submitted in partial fulfillment of require ments for an advanced degree at the University of Arizona and is deposited in the University Library to be made available to borrowers under rules of the Library. Brief quotations from this thesis are allowable without special permission, provided that accurate acknowledgment of source is made. Requests of permission for extended quotation from or reproduction of this manuscript in whole or in part may be granted by the head of the major department or the Dean of the Graduate College when in their judg ment the proposed use of the material is in the interests of scholarship. -

The Effect of Light Intensity and Tidal Cycle on the Hatching and Larval Behaviour of the Muricid Gastropod Chorus Giganteus
Journal of Experimental Marine Biology and Ecology 440 (2013) 69–73 Contents lists available at SciVerse ScienceDirect Journal of Experimental Marine Biology and Ecology journal homepage: www.elsevier.com/locate/jembe The effect of light intensity and tidal cycle on the hatching and larval behaviour of the muricid gastropod Chorus giganteus José A. Gallardo a,⁎, Antonio Brante b, Juan Miguel Cancino b a Pontificia Universidad Católica de Valparaíso, Avenida Altamirano 1480, Valparaíso, Chile b Departamento de Ecología, Facultad de Ciencias, Universidad Católica de la Santísima Concepción, Casilla 297, Concepción, Chile article info abstract Article history: Encapsulation is a common strategy observed among marine caenogastropods. Although the capsule confers Received 20 August 2012 protection for embryos, its rigidity and strength pose a significant challenge as the larvae hatch. The factors Received in revised form 19 November 2012 that drive hatching among these benthic marine gastropods have scarcely been studied. In this study, we exper- Accepted 22 November 2012 imentally evaluated whether the capsule plug opening and larval release processes were synchronised with day/ Available online xxxx night or tidal cycles in the muricid gastropod Chorus giganteus. In addition, we tested the effect of different levels of light intensity on the swimming behaviour of pre- and post-hatching larvae. The results showed that capsule Keywords: Chorus giganteus plug rupture occurred in synchronous pulses of capsule groups during both day and night. A periodogram anal- Day/night cycle ysis did not show circatidal rhythmicity in either the plug rupture or total number of capsules hatched. The Hatching highest percentage of larvae hatched at sunset and during the night (82.9%), whereas only 17% of them hatched Intracapsular development during the day. -
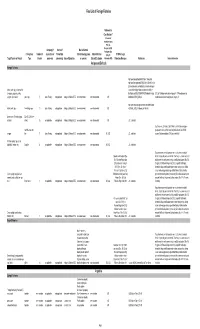
2018 Final LOFF W/ Ref and Detailed Info
Final List of Foreign Fisheries Rationale for Classification ** (Presence of mortality or injury (P/A), Co- Occurrence (C/O), Company (if Source of Marine Mammal Analogous Gear Fishery/Gear Number of aquaculture or Product (for Interactions (by group Marine Mammal (A/G), No RFMO or Legal Target Species or Product Type Vessels processor) processing) Area of Operation or species) Bycatch Estimates Information (N/I)) Protection Measures References Detailed Information Antigua and Barbuda Exempt Fisheries http://www.fao.org/fi/oldsite/FCP/en/ATG/body.htm http://www.fao.org/docrep/006/y5402e/y5402e06.htm,ht tp://www.tradeboss.com/default.cgi/action/viewcompan lobster, rock, spiny, demersal fish ies/searchterm/spiny+lobster/searchtermcondition/1/ , (snappers, groupers, grunts, ftp://ftp.fao.org/fi/DOCUMENT/IPOAS/national/Antigua U.S. LoF Caribbean spiny lobster trap/ pot >197 None documented, surgeonfish), flounder pots, traps 74 Lewis Fishing not applicable Antigua & Barbuda EEZ none documented none documented A/G AndBarbuda/NPOA_IUU.pdf Caribbean mixed species trap/pot are category III http://www.nmfs.noaa.gov/pr/interactions/fisheries/tabl lobster, rock, spiny free diving, loops 19 Lewis Fishing not applicable Antigua & Barbuda EEZ none documented none documented A/G e2/Atlantic_GOM_Caribbean_shellfish.html Queen conch (Strombus gigas), Dive (SCUBA & free molluscs diving) 25 not applicable not applicable Antigua & Barbuda EEZ none documented none documented A/G U.S. trade data Southeastern U.S. Atlantic, Gulf of Mexico, and Caribbean snapper- handline, hook and grouper and other reef fish bottom longline/hook-and-line/ >5,000 snapper line 71 Lewis Fishing not applicable Antigua & Barbuda EEZ none documented none documented N/I, A/G U.S. -

Brief Glossary and Bibliography of Mollusks
A Brief Glossary of Molluscan Terms Compiled by Bruce Neville Bivalve. A member of the second most speciose class of Mollusca, generally bearing a shell of two valves, left and right, and lacking a radula. Commonly called clams, mussels, oysters, scallops, cockles, shipworms, etc. Formerly called pelecypods (class Pelecypoda). Cephalopoda. The third dominant class of Mollusca, generally without a true shell, though various internal hard structures may be present, highly specialized anatomically for mobility. Commonly called octopuses, squids, cuttles, nautiluses. Columella. The axis, real or imaginary, around and along which a gastropod shell grows. Dextral. Right-handed, with the aperture on the right when the spire is at the top. Most gastropods are dextral. Gastropod. A member of the largest class of Mollusca, often bearing a shell of one valve and an operculum. Commonly called snails, slugs, limpets, conchs, whelks, sea hares, nudibranchs, etc. Mantle. The organ that secretes the shell. Mollusk (or mollusc). A member of the second largest phylum of animals, generally with a non-segmented body divided into head, foot, and visceral regions; often bearing a shell secreted by a mantle; and having a radula. Operculum. A horny or calcareous pad that partially or completely closes the aperture of some gastropodsl. Periostracum. The proteinaceous layer covering the exterior of some mollusk shells. Protoconch. The larval shell of the veliger, often remains as the tip of the adult shell. Also called prodissoconch in bivlavles. Radula. A ribbon of teeth, unique to mollusks, used to procure food. Sinistral. Left-handed, with the aperture on the left when the spire is at the top. -

Pennsylvanian Crinoids of New Mexico
Pennsylvanian crinoids of New Mexico Gary D. Webster, Department of Geology, Washington State University, Pullman, WA 99164, and Barry S. Kues, Department of Earth and Planetary Sciences, University of New Mexico, Albuquerque, NM 87131 Abstract north of Alamogordo; (2) Morrowan and groups present on the outcrop were sam- Atokan specimens from the La Pasada For- pled, often repeatedly over the years. Crinoids from each of the five Pennsylvanian mation in the Santa Fe area; and (3) a mid- These collections, reposited at the Univer- epochs are described from 26 localities in dle Desmoinesian species from the Alami- sity of New Mexico (UNM), testify to the New Mexico. The crinoid faunas occupied tos Formation, north of Pecos. Bowsher rarity of identifiable crinoid cups and diverse shelf environments around many intermontane basins of New Mexico during and Strimple (1986) described 15 late crowns in these assemblages, even in cases the Pennsylvanian. The crinoids described Desmoinesian or early Missourian crinoid where crinoid stem elements are common. here include 29 genera, 39 named species, species (several not named) from near the In nearly all collections, crinoid cups rep- and at least nine unnamed species, of which top of the Bug Scuffle Member of the Gob- resent only a small fraction of 1% of the one genus and 15 named species are new. bler Formation south of Alamogordo in the total identifiable specimens. The only This report more than doubles the number of Sacramento Mountains. Kietzke (1990) exception is the fauna from the Missourian previously known Pennsylvanian crinoid illustrated a Desmoinesian microcrinoid Sol se Mete Member, Wild Cow Formation, species from New Mexico; 17 of these species from the Flechado Formation near Talpa. -

The Fossil Record of Shell-Breaking Predation on Marine Bivalves and Gastropods
Chapter 6 The Fossil Record of Shell-Breaking Predation on Marine Bivalves and Gastropods RICHARD R. ALEXANDER and GREGORY P. DIETL I. Introduction 141 2. Durophages of Bivalves and Gastropods 142 3. Trends in Antipredatory Morphology in Space and Time .. 145 4. Predatory and Non-Predatory Sublethal Shell Breakage 155 5. Calculation ofRepair Frequencies and Prey Effectiveness 160 6. Prey Species-, Size-, and Site-Selectivity by Durophages 164 7. Repair Frequencies by Time, Latitude, and Habitat.. 166 8. Concluding Remarks 170 References 170 1. Introduction Any treatment of durophagous (shell-breaking) predation on bivalves and gastropods through geologic time must address the molluscivore's signature preserved in the victim's skeleton. Pre-ingestive breakage or crushing is only one of four methods of molluscivory (Vermeij, 1987; Harper and Skelton, 1993), the others being whole organism ingestion, insertion and extraction, and boring. Other authors in this volume treat the last behavior, whereas whole-organism ingestion, and insertion and extraction, however common, are unlikely to leave preservable evidence. Bivalve and gastropod ecologists and paleoecologists reconstruct predator-prey relationships based primarily on two, although not equally useful, categories of pre-ingestive breakage, namely lethal and sublethal (repaired) damage. Peeling crabs may leave incriminating serrated, helical RICHARD R. ALEXANDER • Department of Geological and Marine Sciences, Rider University, Lawrenceville, New Jersey, 08648-3099. GREGORY P. DIETL. Department of Zoology, North Carolina State University, Raleigh, North Carolina, 27695-7617. Predator-Prey Interactions in the Fossil Record, edited by Patricia H. Kelley, Michal Kowalewski, and Thor A. Hansen. Kluwer Academic/Plenum Publishers, New York, 2003. 141 142 Chapter 6 fractures in whorls of high-spired gastropods (Bishop, 1975), but unfortunately most lethal fractures are far less diagnostic of the causal agent and often indistinguishable from abiotically induced, taphonomic agents ofshell degradation. -

Mollusca, Archaeogastropoda) from the Northeastern Pacific
Zoologica Scripta, Vol. 25, No. 1, pp. 35-49, 1996 Pergamon Elsevier Science Ltd © 1996 The Norwegian Academy of Science and Letters Printed in Great Britain. All rights reserved 0300-3256(95)00015-1 0300-3256/96 $ 15.00 + 0.00 Anatomy and systematics of bathyphytophilid limpets (Mollusca, Archaeogastropoda) from the northeastern Pacific GERHARD HASZPRUNAR and JAMES H. McLEAN Accepted 28 September 1995 Haszprunar, G. & McLean, J. H. 1995. Anatomy and systematics of bathyphytophilid limpets (Mollusca, Archaeogastropoda) from the northeastern Pacific.—Zool. Scr. 25: 35^9. Bathyphytophilus diegensis sp. n. is described on basis of shell and radula characters. The radula of another species of Bathyphytophilus is illustrated, but the species is not described since the shell is unknown. Both species feed on detached blades of the surfgrass Phyllospadix carried by turbidity currents into continental slope depths in the San Diego Trough. The anatomy of B. diegensis was investigated by means of semithin serial sectioning and graphic reconstruction. The shell is limpet like; the protoconch resembles that of pseudococculinids and other lepetelloids. The radula is a distinctive, highly modified rhipidoglossate type with close similarities to the lepetellid radula. The anatomy falls well into the lepetelloid bauplan and is in general similar to that of Pseudococculini- dae and Pyropeltidae. Apomorphic features are the presence of gill-leaflets at both sides of the pallial roof (shared with certain pseudococculinids), the lack of jaws, and in particular many enigmatic pouches (bacterial chambers?) which open into the posterior oesophagus. Autapomor- phic characters of shell, radula and anatomy confirm the placement of Bathyphytophilus (with Aenigmabonus) in a distinct family, Bathyphytophilidae Moskalev, 1978.